Introduction
During orthodontic bonding procedures, excess adhesive is invariably left on the tooth surface at the interface between the bracket and the enamel junction; it is called excess adhesive flash (EAF). We comparatively evaluated the biofilm formation of Streptococcus mutans on EAF produced by 2 adhesives and examined the therapeutic efficacy of xylitol on S mutans formed on EAF.
Methods
First, we investigated the biofilm formation of S mutans on 3 orthodontic bracket types: stainless steel preadjusted edgewise, ceramic preadjusted edgewise, and stainless steel self-ligating. Subsequently, tooth-colored Transbond XT (3M Unitek, Monrovia, Calif) and green Grengloo (Ormco, Glendora, Calif) adhesives were used for bonding ceramic brackets to extracted teeth. S mutans biofilms on EAF produced by the adhesives were studied using the crystal violet assay and scanning electron microscopy. Surface roughness and surface energy of the EAF were examined. The therapeutic efficacies of different concentrations of xylitol were tested on S mutans biofilms.
Results
Significantly higher biofilms were formed on the ceramic preadjusted edgewise brackets ( P = 0.003). Transbond XT had significantly higher S mutans biofilms compared with Grengloo surfaces ( P = 0.007). There was no significant difference in surface roughness between Transbond XT and Grengloo surfaces ( P >0.05). Surface energy of Transbond XT had a considerably smaller contact angle than did Grengloo, suggesting that Transbond XT is a more hydrophilic material. Xylitol at low concentrations had no significant effect on the reduction of S mutans biofilms on orthodontic adhesives ( P = 0.016).
Conclusions
Transbond XT orthodontic adhesive resulted in more S mutans biofilm compared with Grengloo adhesive on ceramic brackets. Surface energy seemed to play a more important role than surface roughness for the formation of S mutans biofilm on EAF. Xylitol does not appear to have a therapeutic effect on mature S mutans biofilm.
Highlights
- •
Transbond XT adhesive resulted in more Streptococcus mutans biofilm than did Grengloo adhesive on ceramic brackets.
- •
Surface energy and hydrophilicity seemed to play more important roles than surface roughness for the formation of S mutans biofilm on excess adhesive flash.
- •
Xylitol does not appear to have a therapeutic effect on mature S mutans biofilm.
- •
A novel ex-vivo extracted tooth model system was used.
Patients undergoing fixed orthodontic treatment face a real risk of enamel demineralization attributed to the difficulty of cleaning the minute areas of these appliances. Inaccessible areas of fixed orthodontic appliances may promote formation of dental plaque biofilm and its pathological consequences such as white spot lesions and dental caries. Hence, prevention of dental caries is important for patients having fixed orthodontic treatment. Many studies have shown that Streptococcus mutans , a key bacterial pathogen in dental caries, increases in dental plaque biofilm during orthodontic treatment resulting in greater incidences of demineralization and dental caries.
Clinically, common sites for demineralization are at the orthodontic adhesive and enamel junction, surrounding the tooth structure of the bracket base. During bonding procedures, there is a certain amount of adhesive left on the tooth surface along the margin between the bracket and the enamel interface. This is called excess adhesive flash (EAF). If it is not properly removed, the rough adhesive surface of the EAF region provides a site for rapid attachment and growth of oral microorganisms. Hence, EAF acts as an anchor area for dental plaque that may harbour pathogenic bacteria such as S mutans .
Orthodontic adhesives are classified into several groups: conventional composites, glass ionomer cements, resin modified glass ionomer cements, and polyacid modified composites. Of these, composite resin adhesives are favored because of their better bonding characteristics. The use of colored orthodontic composite resin adhesive has been suggested to provide better visualization of adhesive, thus facilitating the removal of EAF during orthodontic bonding procedures. Hence, it is assumed that colored composite resin is beneficial for the management of potential enamel demineralization since it prevents the accumulation of excessive dental plaque biofilm near orthodontic brackets. Previous studies have shown that surface characteristics of orthodontic adhesives may significantly affect the adhesion of S mutans to the surface.
The use of external anticaries agents has been shown to be beneficial for the management of dental caries during orthodontic treatment. Xylitol is an alternative sweetener that has been used as an anticariogenic agent during orthodontic treatment. Positive effects of xylitol on caries management have been shown in clinical studies involving young children. Xylitol is available commercially in the form of chewing gum, mouth rinses, and lozenges in various concentrations. In-vitro studies have shown that xylitol has an inhibitory effect of S mutans growth. However, there is no consensus on the most efficient or the minimal therapeutic dose of xylitol. On the other hand, some studies do not agree that xylitol reduces the S mutans levels. Clinical studies with different study designs, administration forms, and dosages have shown inconclusive findings on the effects of xylitol.
To select an appropriate model of orthodontic bracket, first we investigated the biofilm formation of S mutans on 3 bracket types: stainless steel preadjusted edgewise, ceramic preadjusted edgewise, and stainless steel self-ligating. Subsequently, we selected the ceramic orthodontic bracket model to comprehensively examine the effect of different composite adhesives for the adhesion and biofilm formation of S mutans . Next, we evaluated the effect of xylitol on adhesion and biofilm formation of S mutans on the EAF region.
Material and methods
For the first part of the study, extracted premolars (n = 21) were used to examine the effect of 3 orthodontic bracket types—stainless steel preadjusted edgewise, ceramic preadjusted edgewise, and stainless steel self-ligating—on biofilm formation of S mutans . Ethical approval for the use of the teeth was obtained from the institutional review board at National University of Singapore (reference code B-14-010E). Extracted maxillary and mandibular premolars, with no cavitations or cracks on the buccal surfaces of the crowns, and intact buccal surfaces were used for the study. Premolars with restorations, decalcifications, or cracks on the buccal surface of the crown were excluded. The teeth were stored in distilled water. Immediately before bonding, they were cleaned with an ultrasonic scaler and pumice to remove soft-tissue remnants, calculus, and plaque. They were fixed on a Frasaco mounting (MSA, Greenville, NC) with utility wax, in the phantom head, to simulate actual clinical bonding. Then the teeth were randomly separated into 3 groups of 7 teeth each. All teeth were bonded with Transbond XT adhesive (3M Unitek, Monrovia, Calif). The first group was bonded with stainless steel preadjusted edgewise brackets (Gemini; 3M Unitek), the second group with ceramic preadjusted edgewise brackets (Clarity; 3M Unitek), and the third group with stainless steel self-ligating brackets (Damon Q; Ormco, Glendora, Calif).
For the second part of the study, ceramic orthodontic brackets were used, depending on the results of the first part of the study. Hence, extracted premolars (n = 40) were used to examine biofilm formation on the EAF region in this ceramic bracket model. Fourteen premolars were used for the biofilm experiment, 16 were used for the xylitol experiment, and 10 were used for the surface roughness test. This study was approved by the institutional review board of the National University of Singapore (reference number, B-14-0120).
S mutans (strain, ATCC 35668) was obtained from the archival collection of Oral Sciences, Faculty of Dentistry, National University of Singapore. Frozen isolates were thawed, and the identity was reconfirmed using standard methodology. Bacterial stock was recovered on mitis salivarius agar (BD Science, Singapore) aerobically at 37°C. A single colony on mitis salivarius agar was inoculated in sterile brain-heart infusion (Sigma, Singapore) broth. The culture was incubated aerobically at 37°C and shaken at 80 rpm for 16 hours. For the biofilm experiments, the bacterial density was adjusted to 10 8 colony-forming units per milliliter (equivalent to 0.5 McFarland standard at 600 nm).
Immediately before bonding, the teeth were cleaned and mounted on the Frasaco models. Next, the teeth were stabilized in the arch by red utility wax to simulate actual bonding conditions. All brackets were bonded to the teeth according to the manufacturer’s instructions. Excess adhesive was removed with an explorer before it was polymerized. A Satelec mini LED curing light (KaVo, Charlotte, NC) with a 10-mm diameter light tip was used for curing the specimens for 20 seconds at 1100 mW/cm 2 . The crowns of the extracted teeth with bonded brackets were sectioned from their roots and autoclaved to sterilize. The crowns were then transferred to and stabilized in a 24-well plate for the biofilm experiments. The teeth were randomly separated into 6 groups for 2 sets of experiments.
For the first part of the study, teeth with bonded brackets were sectioned, and the crowns (the samples) were autoclaved to sterilize. To specifically develop S mutans biofilms on brackets and tooth surfaces near the brackets, the crowns were fixed onto a 24-well plate (Greiner Bio-One, Kremsmünster, Austria) platform. The assemblies (crown models) were sterilized with ultraviolet light before the experiments. The S mutans biofilm formation protocol was adapted from that of Islam et al with some modifications. A single colony from the mitis salivarius agar was inoculated in sterile brain-heart infusion broth at 37°C for 16 hours. Bacterial culture was adjusted to the inoculum of 10 8 colony-forming units per milliliter (equivalent to optical density 600, or 0.5). The culture was then exposed to the crowns stabilized in a 24-well plate platform, placed at 37°C, and shaken at 80 rpm for 24 hours. Biofilm biomass was quantified using colony-forming unit counting and crystal violet assays. A scanning electron microscope was also used for visualization of the biofilm.
Depending on the first part of the study, the ceramic orthodontic brackets were selected as the model for the second part of the study. Fourteen teeth were used to examine the S mutans biofilm formation on the adhesives. The first group (n = 7) with preadjusted edgewise premolar ceramic brackets (Clarity) was bonded by the standard protocol of etching with 37% phosphoric acid and application of Transbond XT light cure orthodontic primer followed by Transbond XT composite adhesive paste on ceramic brackets. The second group (n = 7) of preadjusted edgewise premolar ceramic brackets (Clarity) was bonded by the standard protocol of etching with 37% phosphoric acid and application of Ortho Solo light cure orthodontic primer (Ormco) followed by Grengloo adhesive composite paste (Ormco) on the ceramic brackets.
In the second part of the experiment, the therapeutic effect of xylitol on S mutans biofilm formation was examined using different concentrations of xylitol. Preadjusted standard edgewise premolar ceramic brackets (Clarity) were bonded by the standard protocol of etching with 37% phosphoric acid and application of Transbond XT light-cure orthodontic primer followed by Transbond XT adhesive paste. S mutans biofilms were formed on the samples after 24 hours. Thereafter, 16 teeth were divided into 4 groups (n = 4 for each): a control group that only received the medium, and the 3 test groups that were exposed to 1%, 5%, or 10% xylitol for 1 hour, respectively.
The bacteria from the biofilm biomass were removed mechanically using a pipette tip followed by vortexting in phosphate-buffered saline. Next, phosphate-buffered saline was removed by centrifuging at 5000 rpm for 5 minutes and the bacterial cells in the pellet were stained with 1% crystal violet (Sigma, Saint Louis, Mo). The crystal violet assay was performed as previously described. In brief, excess crystal violet was removed, and 95% ethylic alcohol (Merck, Singapore) was applied to resolubilize the dye bounded to biofilms. Solutions obtained were transferred to a new sterile flat-bottom 96-well plate, and the optical density of the content was measured using a microtiter plate spectrophotometer (μQuan Microplate spectrophotometer; BioTek, Winooski, Vt) at 570 nm.
Scanning electron microscopy (XL30CP; Philips, Amsterdam, Netherlands) was used to visualize the complex structure of the biofilms on the brackets. Specimens with biofilm exposure were subsequently washed to remove nonadhered bacteria in distilled water, fixed in 2.5% glutaraldehyde (Sigma, Singapore) overnight at 4°C, dehydrated gently by washing in an ethanol alcohol series (70% for 1 hour, 95% for 10 minutes, and 100% for 10 minutes), and air dried in a desiccator before sputter coating with gold. Afterward, the specimens were mounted on the aluminum stubs and coated with gold in a low-pressure atmosphere with an ion sputter coater (JFC1 100; Jeol, Tokyo, Japan). The topographic features of the biofilm were visualized with the scanning electron microscope.
Surface features of the EAF region were characterized using surface roughness and surface free energy as parameters. Surface roughness of the EAF surrounding the brackets was measured using Rugosimeter device (SJ 400; Mitutoyo, Tokyo, Japan). Five teeth with each type of adhesive were used. The analysis followed ISO 4287-1997 standards, with Gaussian filter and the cutoff wavelength value of 0.8 mm. Four surface roughness measurements were taken for each sample.
Surface free energy of the EAF was measured by the sessile drop method using deionized water as previously described. A video camera equipped with an image analyzer (Phoenix 300; Surface Electro Optics, Seoul, Korea) visualized the shape of the drop to provide a measure of the contact angle. The contact angle measurements were made at room temperature. The volume of the drop was controlled by a machine interfaced with a computer. The image of the liquid droplet was obtained in real time acquisition using a CCD camera. Right and left contact angles of each drop were automatically calculated from the computer and averaged to give 1 contact angle per drop. Each experiment was repeated 5 times for 3 specimens of each material.
Statistical analysis
The statistical analysis was performed with the Kruskal-Wallis test; for any pairwise comparisons, the Mann-Whitney U test was used with the Bonferroni adjustment. All analyses were done with software (version 16.0; SPSS, Chicago, Ill). The level of statistical significance was set at P >0.05.
Results
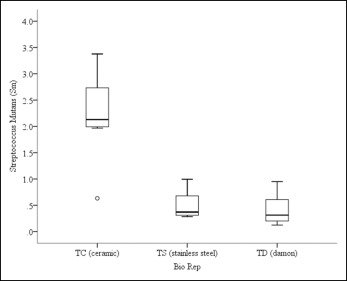
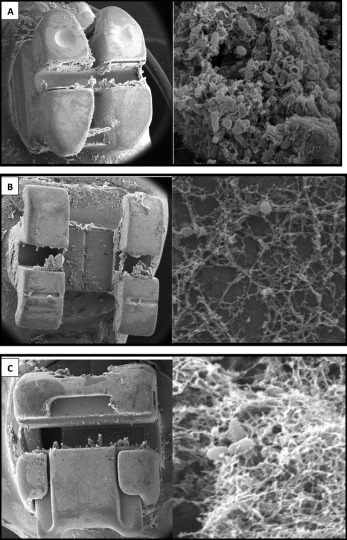
The crystal violet assay showed statistically significant differences in S mutans biofilm formation among the 3 types of orthodontic brackets ( P = 0.003) ( Fig 1 ). Significantly higher biofilm colonization was observed on the ceramic edgewise brackets, compared with the stainless steel edgewise brackets ( P = 0.012) and the stainless steel self-ligating brackets ( P = 0.012). There was no significant difference in the bacterial biofilms between the stainless steel edgewise brackets and the stainless steel self-ligating brackets ( P >0.05). Scanning electron microscope images corroborated the in-vitro observation ( Fig 2 ). Significantly higher biofilms consisting of S mutans cells embedded in a polysaccharide matrix were observed on the ceramic edgewise brackets compared with the meager biofilms on the stainless steel edgewise brackets and the stainless steel self-ligating brackets. Therefore, the ceramic edgewise brackets were selected for the second part of the study.
S mutans biofilm evaluated by the crystal violet assay demonstrated that the Transbond XT composite adhesive group has significantly higher biofilms compared with the Grengloo composite adhesive ( P = 0.007) ( Fig 3 ). Scanning electron microscope observation of the biofilm-free adhesive surfaces clearly showed that Transbond XT adhesive had more EAF remnants surrounding the brackets than did the Grengloo adhesive ( Fig 4 ).




