Fig. 1.1
Illustration of the masseter muscle and temporalis muscle
1.2.2.2 Temporalis Muscle
The temporalis muscle is a major mandibular elevator of the mandible and consists of three separate muscular components having different force vector orientation and displaying a distinct “fan shape.” The temporalis muscle has its origin at the lateral aspect of the skull and envelopes nearly the entire temporal fossa as the anterior, medial, and posterior temporalis fibers. All three fibers then merge and course inferiorly through the medial aspect of the zygomatic process where they converge by way of the temporalis tendon at the coronoid process of the mandible and extend to the anterior border of the mandibular ramus. The anterior fibers are aligned vertically while the posterior fibers are essentially horizontal and the middle fibers are at an oblique or diagonal orientation (“fan shaped”). Functionally, the three distinct fibers are all involved in vertical closure (elevation) of the mandible. The anterior fibers are primarily involved in closure (mandibular elevation to tooth contact). The posterior fibers are involved in closure and seating of the condyle/disc complex during that movement as well as limited retrusion of the mandible after it has been protruded. All three fibers also play a role in lateral excursive movement of the mandible (ipsilateral lateral excursive movement) [9, 20]. The temporalis muscle has both sensory and motor innervation supplied by the deep temporal branches of the trigeminal nerve (V3). Contributions to its vascular supply are from branches of the maxillary artery: the anterior deep temporal artery supplies approximately 20 % of the anterior temporalis, the posterior deep temporal artery supplies approximately 40 % of the posterior temporalis, and the remaining 40 % (middle temporalis muscle) is supplied by the middle temporal artery (See Fig. 1.1).
1.2.2.3 Medial Pterygoid Muscle
The medial pteryoid or “internal pterygoid” muscle is a pennated masticatory muscle having two distinct points of origin. The deep head originates from the medial surface of the lateral pterygoid plate of the sphenoid. A smaller superficial head arises from the maxillary tuberosity and pyramidal process of the palatine bone. These fibers are oriented in a posterior and inferior fashion and share a tendinous insertion at the medial surface of the ramus and angle of the mandible at or near the insertion of the masseter muscle (pterygomasseteric sling). Functionally, its primary roles are in closure of the mandible (mandibular elevation), contralateral deviation, and, in conjunction with the lateral pterygoids, protrusion of the mandible. Contraction of ipsilateral medial pterygoid and lateral pterygoid muscles results in contralateral lateroexcursive movement of the mandible with translatory movement of the ipsilateral condyle and rotational movement of the contralateral condyle or contralateral deviation [9, 20].
Additionally, the medial pterygoid muscle is often described as a “functional analog” of the masseter muscle in terms of function and alignment, and is also closely associated with fibers of the tensor veli palatini muscle. This anatomical relationship of the medial pterygoid muscle and the tensor veli palatini muscle has been the subject of several studies examining the possible role of both muscles in the development of eustachian tube-related functional problems including patulous (open) eustachian tube and auditory tube dysfunction [9, 20, 21]. It has been theorized that the function of the medial pterygoid muscle moderates or influences the opening pressure of the auditory tube, and this in turn has been associated with “ear fullness” complaints in patients reporting with ear-related TMD symptoms in which hyperactivity of the elevator muscles is suspected relative to pain complaints [20, 22, 23]. Vascular supply of the medial pterygoid muscle is from the pterygoid branches of the maxillary artery, and it receives its sensory and motor innervation from the third branch (V3) of the trigeminal nerve (medial pterygoid branch) (See Fig. 1.2).
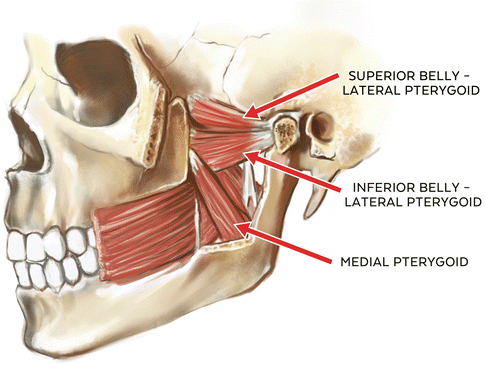

Fig. 1.2
Illustration of the medial pterygoid muscle and lateral pterygoid muscle (superior and inferior bellies)
1.2.2.4 Lateral Pterygoid Muscle
It is a nonpennated muscle of mastication that is involved in both mandibular depression and elevation. It consists of two independent “heads” or “bellies” (superior and inferior) that have independent functional roles in mandibular movement [24]. The superior portion has its origin at the infratemporal surface and infratemporal crest of the greater wing of the sphenoid bone. Its fibers primarily insert at the anterior fovea (pterygoid fovea) of the mandibular condyle, with a variable secondary insertion occurring at the temporomandibular joint disc/capsule complex as well as medial and anterior portions of the disc. Approximately 60–70 % of the fibers insert at the anterior fovea of the condyle while 30–40 % insert at the capsule-disc complex [11, 20]. While it has been posited that the superior lateral pterygoid muscle can pull the disc anteriorly from its contact with the condyle, this does not seem to be anatomically possible. The inferior portion of the lateral pterygoid has a less diverse origin-insertion identity as does the superior portion, and has its origin at the outer surface of the lateral pterygoid plate and insertion at the anterior fovea and neck of the condyle.
Functionally, the lateral pterygoid muscles are considered to play a major role in protrusion and contralateral deviation of the mandible [9]. Specifically, the upper or superior belly is active as a counterbalance or disc/condyle stabilizer during closure of the mandible while the inferior or lower belly of the lateral pterygoid is significantly inhibited during this movement. Conversely, the inferior belly is active in opening and protrusion of the mandible while the superior belly is significantly inhibited during this movement, giving the lateral pterygoid muscle a very unique role in mandibular movement based upon independent functions of the superior and inferior bellies of this masticatory muscle [24].
While the bellies of the lateral pteryoid muscle are active as depressor and elevator muscles in mandibular movement, overall, the lateral pterygoid is considered to be of secondary importance in mandibular opening movement, with the digastric and geniohyoid muscles being the primary mandibular depressors (opening) [20]. Due to the unique anterior and medial alignment of the lateral pterygoid fibers to the bilateral mandibular condyles, bilateral activation of the inferior bellies of the pterygoids results in a forward or protrusive movement of the mandible having an anterior or horizontal projection, especially during parafunctional activities and heavy mastication [27]. However, unilateral activation of either inferior belly will result in a shift of the mandible to the ipsilateral side and a resulting translational movement of the contralateral side; thus, the terms “condylar translation” and “condylar rotation.” This movement is observed in both functional lateral excursion of the mandible (chewing stroke) as in mastication and in parafunctional lateral excursive movement of the mandible as in bruxing and clenching. In conjunction with the digastric and geniohyoid muscles, the lateral pterygoids play a significant role in the three-dimensional functional envelope (Posselt’s Envelope of Motion) of mandibular movement in vertical, anterior-posterior, and lateral (transverse) dimensions. Since the origins of lateral pterygoid muscles are medial to their insertions, wide opening of the mandible may result in the mandible being temporarily distorted transversely, with the bilateral posterior lingual borders actually being distorted toward the midline. Thus, taking an impression of the lower dentition with the mouth widely open may result in study casts that are not accurate in the transverse dimension (Mohl, N.D., Verbal communication) [44]. Unlike the other jaw closing muscles, the lateral pterygoids are unique in that they do not contain muscle spindles. The absence of muscle spindles may help to explain why the lateral pterygoids play a secondary role in mandibular depression, where stretch receptors (muscle spindles) are essential for detecting muscle working length change and velocity, and help prevent excessive stretching of the muscle during functional movement [14, 46].
The lateral pteryogid muscles also act in conjunction with the posterior temporalis muscle fibers in controlling anterior and posterior translation of the mandible [9]. For a more detailed explanation of the functional movement of the mandible relative to mastication, the reader may refer to Functional Biomechanics of the Masticatory System: in Management of Temporomandibular Disorders and Occlusion, 7th edition, Chapter 1, p. 13, and Mechanics of Mandibular Movement: in Management of Temporomandibular Disorders and Occlusion, 7th edition, Chapter 4, pp. 62–72 Okeson, J.
Vascular supply of both heads of the lateral pterygoid muscles is from the pterygoid branches of the maxillary artery and from the ascending palatine branch of the facial artery. Its unique “independent function” mirrors its superior and inferior belly innervation. That is, while the primary innervation is from the third division of the trigeminal nerve (V3 or mandibular nerve), the superior belly and the lateral fibers of the inferior belly receive their innervation from the buccal branch of the mandibular nerve (V3) and the medial fibers of the inferior belly receive their innervation from the anterior trunk of the mandibular nerve (V3) [20] (See Fig. 1.2).
1.2.2.5 Digastric Muscle
The digastric muscle, like the lateral pterygoid muscle, has two distinct components: the anterior and posterior belly, neither of which contains muscle spindles. The anterior belly has its origin at the digastric fossa and submental area near the midline, and its fibers extend inferiorly and posteriorly. The posterior belly has its origin at the mastoid notch of the temporal bone, and its fibers extend inferiorly and forward to join with the anterior belly at a common intermediate tendon attachment at the body and greater horn of the hyoid bone. Both bellies of the digastric muscle act to depress the mandible (opening), and the posterior belly is also involved in elevating the hyoid bone during mastication and swallowing. One important variant in the origin of the anterior belly is that its fibers may cross the anatomical midline at the submental area, and it has been proposed that this variation may play a role in detectable but nonpathologic mandibular deviation on opening [25].Vascular supply to the anterior belly is from the submental branch of the facial artery. The posterior belly receives its blood supply from the posterior auricular and occipital arteries. An interesting and unique feature of the digastric muscle is in its innervation; that is, the anterior belly is innervated by the mylohyoid branch of the inferior alveolar nerve (V3) while the posterior belly is innervated by the facial nerve (VII), highlighting their distinct and separate derivations from 1st and 2nd branchial arches [26] (See Fig. 1.3).
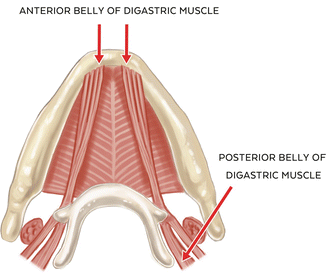

Fig. 1.3
Illustration of the digastric muscle (anterior and posterior belly)
1.2.3 Functional Characteristics of the Masticatory Muscles
Coordinated and efficient movement of the mandible is a highly complex function of the neuromuscular system primarily involving muscle fibers, sensory and motor nerves, proprioceptive receptors, and a control “center” within the reticular formation of the midbrain. This coordinated effort has both voluntary as well as involuntary characteristics in terms of stimulation, inhibition, and control, and it is within the context of these phenomena that static, functional, and parafunctional movements of the mandible derive their appropriate descriptions.
The primary skeletal muscles of the masticatory system are designated by their function; that is, elevators/retractors (muscles primarily involved in mandibular closing) and depressors/protruders (muscles primarily involved in mandibular opening). As in all skeletal muscles, their functional relationships are closely coordinated with one another so as to ensure a more efficient and coordinated movement. The actual function of the muscle is dependent upon its origin and insertion, with the origin of the muscle being at a stationary location (musculotendinous anchorage) and the insertion being the attachment of the muscle to the body being moved (bone and joint mechanics). Often the muscles of mastication are labeled as being agonists (primary movers) or antagonists (those that oppose a particular movement) depending upon the functional movement that each is going through at a particular point in time. For example, the elevator muscles may be thought of as being active in closing movement of the mandible while the depressor muscles are relaxed, with the opposite being true during opening movement. However, both groups of muscles are functionally active to some degree at all times rather than being sequentially stimulated or totally inhibited and in opposition to the other during mandibular movement. The three-dimensional functional movement of the mandible requires a high degree of coordinated effort between and among both classes of muscle [9, 15].
Another anatomical factor to address is the architectural arrangement of the muscles of mastication relative to their force and power output. Muscles that have their muscle fibers (fascicles) arranged in an oblique orientation to their origin are considered to be pennated with fibers arranged in some type of “feather-like” or “plume-like” arrangement [9, 10, 28]. Pennated muscles have tendons that extend for most of the muscle’s length (origin to insertion), and its fibers are obliquely aligned as they insert into the tendons. Nonpennated muscles have their fascicles arranged in a more parallel-like orientation relative to the origin and insertion of the muscle. The mechanical advantage of the pennated muscles is that they generally possess a greater number of muscle fibers per volume area (physiological cross sectional area or PCSA), resulting in a greater net force production than muscles arranged in parallel orientation. However, while pennated muscles produce more force than parallel muscles, they tend to demonstrate less power output (force x displacement) as a side effect of their orientation as well as less range of motion. Overall, the muscles of mastication have a mixed architectural classification with the masseter, temporalis and medial pterygoid muscles classified as pennated and the lateral pterygoid muscle classified as a nonpennated skeletal muscle [29].
The term “power stroke of mastication” is often used to describe the elevation of the mandible during closure to an at-or-near tooth contact during mastication (“chewing stroke of mastication”) [30]. A single chewing stroke consists of one cycle or loop of mandibular depression, lateral deviation of the mandible, and elevation (“tear drop” configuration in the frontal plane). “Power” is defined as the amount of energy consumed per unit time, is dependent upon the trajectory of the point of force application and torque, is measured in joules per second or watts, and is expressed as “power = work/time.” “Force” is defined as a particular amount of energy being exerted in a specific direction (measured in newtons), and is the more accurate expression for mandibular closure movement (bite registration, for example) rather than “power,” which, by definition, is velocity or movement/second [13, 16]. Therefore, it is proposed that the term “force closure” be substituted for “power closure” in the discussion of masticatory biomechanics, especially when describing those forces exerted during the chewing stroke (opening and closing) and orthodontic bite registration, for example [34, 35].
Coordination of the masticatory cycle is primarily controlled by the central pattern generator (CPG) of mastication, a neurosensory gait or rhythmicity control system located within the reticular formation of the brainstem. It directs involuntary masticatory function with associated input from the thalamus, hypothalamus, and limbic system. Overriding influences created by emotional stress and/or parafunctional activity may also influence the overall CPG-directed coordination of complex masticatory movements [12, 31, 33].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


