Parotid gland tumors can be a challenging clinical entity to manage for the head and neck surgeon. Evaluation of these patients is no different from that for any other patient and should include a thorough history and physical examination followed by appropriate imaging when indicated and, ultimately, tissue biopsy to arrive at a diagnosis. Close collaboration between the referring physician, the head and neck surgeon, and the oncologist is required to ensure optimal patient care. Advances in microscopic diagnosis, imaging, and surgical techniques over the past decade have improved care for patients with salivary gland neoplasms.
Etiopathogenesis
Salivary gland tumors are rare in comparison to the overall incidence of tumors of the head and neck region. The overall incidence of salivary gland tumors varies around the world from approximately 0.4 to 13.5 cases per 100,000 people. The parotid gland is the most common site of occurrence of salivary gland tumors, and in four large series published on primary epithelial salivary gland tumors, the parotid gland accounted for 64% to 80% of all cases ( Table 56-1 ). Only 15% to 32% of these cases were malignant ( Table 56-2 ). The most common benign parotid tumor is pleomorphic adenoma, and the most common malignant parotid tumor is mucoepidermoid carcinoma ( Table 56-3 ). Generally speaking, two thirds to three quarters of salivary gland tumors occur in the parotid and two thirds to three quarters of these tumors are benign.
| AUTHOR | NO. CASES | SITE OF OCCURRENCE | |||
|---|---|---|---|---|---|
| PAROTID | SUBMANDIBULAR | SUBLINGUAL | MINOR | ||
| Ellis et al. | 13,749 | 64% | 10% | 0.3% | 23% |
| Spiro | 2,807 | 70% | 8% | Included with minor | 22% |
| Seifert et al. | 2,579 | 80% | 10% | 1.0% | 9% |
| Eveson and Cawson | 2,410 | 73% | 11% | 0.3% | 14% |
| AUTHOR | NO. CASES | PERCENTAGE OF MALIGNANT CASES | |||
|---|---|---|---|---|---|
| PAROTID | SUBMANDIBULAR | SUBLINGUAL | MINOR | ||
| Ellis et al. | 13,749 | 32% | 41% | 70% | 49% |
| Spiro | 2,807 | 25% | 43% | Included with minor | 82% |
| Seifert et al. | 2,579 | 20% | 45% | 90% | 45% |
| Eveson and Cawson | 2,410 | 15% | 37% | 86% | 46% |
| ELLIS ET AL. | EVESON AND CAWSON | THACKRAY AND LUCAS | ENEROTH | FOOTE AND FRAZELL | |
|---|---|---|---|---|---|
| Total number of cases | 8,222 | 1,756 | 651 | 2,158 | 764 |
| Benign Tumors | |||||
| Pleomorphic adenoma | 53.0% | 63.3% | 72.0% | 76.8% | 58.5% |
| Warthin tumor | 7.7% | 14.0% | 9.0% | 4.7% | 6.5% |
| Oncocytoma | 1.9% | 0.9% | 0.6% | 1.0% | 0.1% |
| Basal cell adenoma | 1.4% | — | — | — | — |
| Other | 3.7% | 7.1% | 1.8% | — | 0.7% |
| Total | 67.7% | 85.3% | 83.4% | 82.5% | 65.8% |
| Malignant Tumors | |||||
| Mucoepidermoid carcinoma | 9.6% | 1.5% | 2.3% | 4.1% | 11.8% |
| Acinic cell carcinoma | 8.6% | 2.5% | 1.2% | 3.1% | 2.7% |
| Adenoid cystic carcinoma | 2.0% | 2.0% | 3.3% | 2.3% | 2.1% |
| Malignant mixed tumor | 2.5% | 3.2% | 4.1% | 1.5% | 6.0% |
| Squamous cell carcinoma | 2.1% | 1.1% | 1.0% | 0.3% | 3.4% |
| Other | 7.5% | 4.4% | 4.7% | 6.3% | 8.1% |
| Total | 32.3% | 14.7% | 16.6% | 17.5% | 34.2% |
Very little is known about the etiology of salivary gland tumors ( Box 56-1 ). The cause is thought to be multifactorial, and many factors have been suggested to play a role. One relationship often cited is between Epstein-Barr virus (and possible autoimmunity) and malignant lymphoepithelial tumors of the parotid. The strongest support for this contention comes from epidemiologic studies of the Eskimo population in Greenland. Merrick and colleagues found that the virus is normally present in the pharynx and salivary glands of this population and that the interrelationships of immunity, the virus, and the genetic composition of the host may all play a role in the malignant transformation of salivary gland epithelial cells.
- •
Viral
- •
Epstein-Barr virus
- •
Human herpesvirus-8
- •
Human papillomavirus
- •
- •
Ionizing Radiation
- •
Occupation
- •
Hairdresser
- •
Asbestos mining
- •
Plumber
- •
Automobile manufacturing
- •
- •
Oncogenes
Dalpa and co-workers investigated the link between human herpesvirus 8 (HHV-8) and Warthin tumors. They evaluated for the presence of HHV-8 in a series of Warthin tumors of the parotid gland and corresponding adjacent normal tissue. HHV-8 DNA was detected in 19 of 43 (44%) salivary gland tumor samples. Among the 15 cases with paired samples, 9 were HHV-8 positive for both samples, 4 were HHV-8 negative for both samples, and in 2 cases HHV-8 was detected only in the tumor specimens. They concluded that HHV-8 is frequently detected in adenoid salivary neoplasms, thus suggesting a significant role of the virus in the etiopathogenesis of the disease.
Vageli and associates examined the relationship between high-risk human papillomavirus (HPV) and parotid tumors. They studied nine parotid lesions for HPV infection, including an oncocytoma, an acinic cell carcinoma, a high-grade adenocarcinoma, a low-grade polymorphous adenocarcinoma, a Warthin tumor, two pleomorphic adenomas, a lymphoepithelial cyst, and a lipoma of the parotid gland. DNA was extracted from formalin-fixed and paraffin-embedded tissue sections. HPV typing was carried out by multiplex polymerase chain reaction (PCR) for HPV genotypes 6, 11, 16, 18, and 33; positive samples were reconfirmed by PCR with specific primers for each type. Quantitative real-time PCR for the high-risk HPV genotypes 16, 18, 31, 33, 35, 52, 58, and 67 was also performed to determine the viral load. Seven of the nine parotid lesions were HPV positive, whereas six of these seven had been infected by the HPV-16 or HPV-18 oncogenic types (or by both). A high viral load of the high-risk genotypes of HPV was found in the oncocytoma, in one of the pleomorphic adenomas, and in the Warthin tumor. Finally, in situ PCR indicated that HPV-16 amplification occurred in the salivary gland tumors. Their results suggest possible involvement of the virus in the disease.
An overwhelming body of literature supports the relationship between parotid tumors and ionizing radiation. Boukheris and colleagues evaluated the magnitude of the risk for the development of salivary gland tumors in patients who had undergone radiotherapy for Hodgkin lymphoma. The risk for salivary gland carcinoma in 20,928 1-year survivors of Hodgkin lymphoma in whom the disease was diagnosed between 1973 and 2003 was evaluated in 11 population-based cancer registry areas of the Surveillance, Epidemiology and End Results (SEER) program. Among 11,047 patients with Hodgkin lymphoma who received radiotherapy as part of their initial treatment, invasive salivary gland carcinoma subsequently developed in 21. The risk for radiation-related salivary gland carcinoma was highest in younger patients (age <20 years) with Hodgkin lymphoma and in 10-year survivors, with risks remaining elevated for at least 2 decades after irradiation. Significant differences in risk by histologic type were observed, with a particularly high risk for the development of mucoepidermoid carcinoma and adenocarcinoma. They concluded that patients treated with radiotherapy experienced a significantly increased risk for salivary gland carcinoma, particularly when exposed at a young age or for at least 2 decades after exposure.
Certain occupations have been shown to increase risk for the development of salivary gland tumors. Swanson and Burns assessed the risk for salivary gland cancers associated with diverse occupations and industries. They carried out a population-based case-referent study using data from a SEER program cancer registry for patients and by telephone interview for patients and referents to evaluate workplace-associated risks for salivary gland cancer in black and white women and men. They found significantly elevated odds ratios in women employed as hairdressers and those working in beauty shops. They concluded that the risk for salivary gland cancer is elevated in women employed as hairdressers.
Other occupations that have been shown to predispose one to the development of salivary gland tumors include asbestos mining, manufacturing of rubber products, plumbing, and the automobile industry.
The role of various oncogenes in salivary gland tumorigenesis has been investigated. Elledge reviewed the literature pertaining to individual oncogenes in which their role as diagnostic and prognostic markers and as potential targets for treatment was discussed. He found articles pertaining to kit, PLAG1, Mect1-Maml2, HMGIC, HER2/neu, ras, c-fos, and Sox-4. All these studies were noted to be seminal small-scale studies with the potential for further research and eventual clinical application. Elledge concluded that a wide variety of oncogenes have been implicated in salivary gland tumorigenesis, with evidence being confined to small murine or in vitro studies. He also concluded that there are possible roles for different oncogenes in therapeutics, prognosis, and management of specific salivary gland tumors.
No association has been found between salivary gland tumors and smoking. Similarly, no association has been found between alcohol consumption and salivary gland neoplasms.
Pathologic Anatomy
Parotid Gland
The parotid gland is the largest of three paired major salivary glands within the head and neck region. It is enclosed within a fascial capsule, the parotidomasseteric fascia, which is derived from the investing layer of the deep cervical fascia. This fascial covering is important from a surgical standpoint in that the overlying skin and subcutaneous tissue can easily be separated from it. The parotid lies within the confines of the parotid bed, which is located anteroinferior to the external acoustic canal. The apex of the parotid gland is posterior to the angle of the mandible, and its base is in close relation to the zygomatic arch. The parotid duct (Stensen duct) passes anteriorly, turns medially at the anterior border of the masseter, pierces the buccinators, and then enters the oral cavity through a small orifice opposite the second maxillary molar. The course of the duct is along a line extending from the base of the earlobe to the vermilion border of the upper lip. Embedded within the substance of the parotid gland in a superficial to deep plane are the parotid plexus, the facial nerve and its branches, the retromandibular vein, and the external carotid artery. On the parotid sheath and within the gland are the parotid lymph nodes.
Gregoire dissected a number of parotid glands from various mammals, including humans, and found that the parotid gland was divided into a deep and a superficial lobe with the facial nerve embedded between the two rather than penetrating the parenchyma. The lateral or superficial lobe is the larger of the two. This is the anatomic basis of parotid surgery that allows preservation of the facial nerve. McWorther reported similar findings but also noted that the two lobes were joined by an isthmus that lies in the middle of the gland anterior to the division of the facial nerve. Furthermore, he found that the two lobes are not always separated by a connective tissue layer.
Facial Nerve
The most crucial anatomic structure to identify and preserve during parotid surgery for benign and some malignant tumors is the facial nerve. The facial nerve has both sensory and motor components. It is the motor nerve to the muscles of facial expression and to the muscles of the scalp, external ear, buccinator, platysma, stapedius, stylohyoid, and posterior belly of the digastric. It supplies special sensory taste to the anterior two thirds of the tongue and general sensation to parts of the external acoustic meatus, soft palate, and adjacent pharynx. It also has a parasympathetic component that supplies secretomotor fibers for the submandibular, sublingual, lacrimal, nasal, and palatine glands.
The facial nerve exits the stylomastoid foramen and gives off three branches before it bifurcates: a branch to the posterior belly of the digastric, the stylohyoid muscle, and the posterior auricular muscles. As it exits the stylomastoid foramen, the facial nerve passes anterior to the posterior belly of the digastric muscle and lateral to the styloid process, external carotid artery, and posterior facial vein and then runs anteriorly for approximately 2 cm before dividing into upper and lower divisions. The nerve is approximately 3 mm in diameter at the point where it exits the stylomastoid foramen and bifurcates. The main trunk is invariably found where the tip of the mastoid process, cartilaginous auditory canal, and superior border of the posterior belly of the digastric muscle meet. Within the parotid gland, it divides at the posterior border of the ramus of the mandible into two primary branches, the cervicofacial (vertically directed, longer and smaller) and the temporofacial (horizontally directed and larger). Both divisions run through the substance of the gland and usually pass over the external jugular vein. After the nerve bifurcates, there are a variable number of tertiary limbs that communicate freely with each other in the periphery. The temporofacial division lies between the superficial and deep lobes of the parotid gland and above the isthmus connecting the two lobes.
Identification of the main trunk of the facial nerve ( Fig. 56-1 ) is the key to safe and successful surgical management of benign and malignant tumors of the parotid gland when preservation of the nerve will not compromise sound oncologic principles. Many techniques have been described and used to identify the main trunk of the facial nerve. The most commonly described surgical landmarks include the tympanomastoid suture (TMS), the posterior belly of the digastric, the transverse process of the axis, the styloid process, and the tragal pointer. Most authors suggest that the TMS line is the most constant and consistent surgical landmark for identification of the main trunk of the facial nerve. It is relatively easy to find and its position is constant. The position of the TMS relative to the main trunk of the facial nerve is very reliable because it leads directly to the stylomastoid foramen, where the nerve can be identified in an area that is not likely to be displaced. On average, the facial nerve is located 2 to 4 mm deep to the medial end of the TMS line.
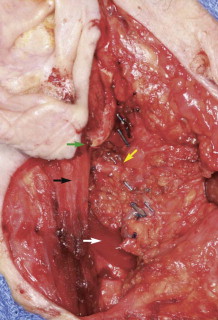
The tragal pointer is another commonly used surgical landmark for identification of the main trunk of the facial nerve but has been found to be somewhat variable in its position relative to the nerve and is therefore less reliable than other landmarks. On average, the nerve is located approximately 1 cm deep and anteroinferior to the tip of the tragal pointer.
The two landmarks commonly cited as being the most reliable and accurate are the posterior belly of the digastric and the TMS line. Witt and colleagues compared the distance from the TMS and posterior belly of the digastric to the main trunk of the facial nerve in both cadavers and patients undergoing parotidectomy. They found the mean distance from the TMS line to the facial nerve to be 1.8 mm (range, 0 to 4 mm) and from the posterior belly of the digastric to the facial nerve to be 12.4 mm (range, 7 to 17 mm) in cadavers. They found the mean distance in live patients from the TMS and posterior belly of the digastric to the main trunk of the facial nerve to be 2.0 mm (range, 0 to 4 mm) and 10.7 mm (range, 5 to 14 mm), respectively. They concluded that the TMS is a significantly closer surgical landmark than the posterior belly of the digastric in both cadaver and live dissections and recommend its use.
Rea and associates had similar findings. They carried out cadaveric dissection of 52 facial halves and found the distance from the posterior belly of the digastric and TMS to the facial nerve to be 5.5 ± 2.1 mm and 2.5 ± 0.4 mm, respectively.
In cases in which the main trunk of the facial nerve cannot be identified, a terminal branch of the nerve can be identified and dissected in retrograde fashion proximally to identify the main trunk. The marginal branch of the facial nerve is the most readily accessible branch for this technique and can normally be safely identified overlying the fascia of the submandibular gland and then traced back toward the main trunk.
Pathologic Anatomy
Parotid Gland
The parotid gland is the largest of three paired major salivary glands within the head and neck region. It is enclosed within a fascial capsule, the parotidomasseteric fascia, which is derived from the investing layer of the deep cervical fascia. This fascial covering is important from a surgical standpoint in that the overlying skin and subcutaneous tissue can easily be separated from it. The parotid lies within the confines of the parotid bed, which is located anteroinferior to the external acoustic canal. The apex of the parotid gland is posterior to the angle of the mandible, and its base is in close relation to the zygomatic arch. The parotid duct (Stensen duct) passes anteriorly, turns medially at the anterior border of the masseter, pierces the buccinators, and then enters the oral cavity through a small orifice opposite the second maxillary molar. The course of the duct is along a line extending from the base of the earlobe to the vermilion border of the upper lip. Embedded within the substance of the parotid gland in a superficial to deep plane are the parotid plexus, the facial nerve and its branches, the retromandibular vein, and the external carotid artery. On the parotid sheath and within the gland are the parotid lymph nodes.
Gregoire dissected a number of parotid glands from various mammals, including humans, and found that the parotid gland was divided into a deep and a superficial lobe with the facial nerve embedded between the two rather than penetrating the parenchyma. The lateral or superficial lobe is the larger of the two. This is the anatomic basis of parotid surgery that allows preservation of the facial nerve. McWorther reported similar findings but also noted that the two lobes were joined by an isthmus that lies in the middle of the gland anterior to the division of the facial nerve. Furthermore, he found that the two lobes are not always separated by a connective tissue layer.
Facial Nerve
The most crucial anatomic structure to identify and preserve during parotid surgery for benign and some malignant tumors is the facial nerve. The facial nerve has both sensory and motor components. It is the motor nerve to the muscles of facial expression and to the muscles of the scalp, external ear, buccinator, platysma, stapedius, stylohyoid, and posterior belly of the digastric. It supplies special sensory taste to the anterior two thirds of the tongue and general sensation to parts of the external acoustic meatus, soft palate, and adjacent pharynx. It also has a parasympathetic component that supplies secretomotor fibers for the submandibular, sublingual, lacrimal, nasal, and palatine glands.
The facial nerve exits the stylomastoid foramen and gives off three branches before it bifurcates: a branch to the posterior belly of the digastric, the stylohyoid muscle, and the posterior auricular muscles. As it exits the stylomastoid foramen, the facial nerve passes anterior to the posterior belly of the digastric muscle and lateral to the styloid process, external carotid artery, and posterior facial vein and then runs anteriorly for approximately 2 cm before dividing into upper and lower divisions. The nerve is approximately 3 mm in diameter at the point where it exits the stylomastoid foramen and bifurcates. The main trunk is invariably found where the tip of the mastoid process, cartilaginous auditory canal, and superior border of the posterior belly of the digastric muscle meet. Within the parotid gland, it divides at the posterior border of the ramus of the mandible into two primary branches, the cervicofacial (vertically directed, longer and smaller) and the temporofacial (horizontally directed and larger). Both divisions run through the substance of the gland and usually pass over the external jugular vein. After the nerve bifurcates, there are a variable number of tertiary limbs that communicate freely with each other in the periphery. The temporofacial division lies between the superficial and deep lobes of the parotid gland and above the isthmus connecting the two lobes.
Identification of the main trunk of the facial nerve ( Fig. 56-1 ) is the key to safe and successful surgical management of benign and malignant tumors of the parotid gland when preservation of the nerve will not compromise sound oncologic principles. Many techniques have been described and used to identify the main trunk of the facial nerve. The most commonly described surgical landmarks include the tympanomastoid suture (TMS), the posterior belly of the digastric, the transverse process of the axis, the styloid process, and the tragal pointer. Most authors suggest that the TMS line is the most constant and consistent surgical landmark for identification of the main trunk of the facial nerve. It is relatively easy to find and its position is constant. The position of the TMS relative to the main trunk of the facial nerve is very reliable because it leads directly to the stylomastoid foramen, where the nerve can be identified in an area that is not likely to be displaced. On average, the facial nerve is located 2 to 4 mm deep to the medial end of the TMS line.
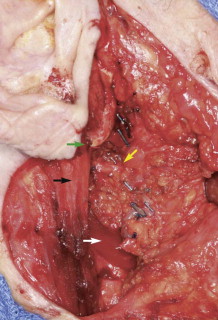
The tragal pointer is another commonly used surgical landmark for identification of the main trunk of the facial nerve but has been found to be somewhat variable in its position relative to the nerve and is therefore less reliable than other landmarks. On average, the nerve is located approximately 1 cm deep and anteroinferior to the tip of the tragal pointer.
The two landmarks commonly cited as being the most reliable and accurate are the posterior belly of the digastric and the TMS line. Witt and colleagues compared the distance from the TMS and posterior belly of the digastric to the main trunk of the facial nerve in both cadavers and patients undergoing parotidectomy. They found the mean distance from the TMS line to the facial nerve to be 1.8 mm (range, 0 to 4 mm) and from the posterior belly of the digastric to the facial nerve to be 12.4 mm (range, 7 to 17 mm) in cadavers. They found the mean distance in live patients from the TMS and posterior belly of the digastric to the main trunk of the facial nerve to be 2.0 mm (range, 0 to 4 mm) and 10.7 mm (range, 5 to 14 mm), respectively. They concluded that the TMS is a significantly closer surgical landmark than the posterior belly of the digastric in both cadaver and live dissections and recommend its use.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


