Abstract
One often alleges that laboratory bond-strength testing cannot predict clinical effectiveness of adhesives. Major argument to sustain this claim is the wide variation in bond-strength values recorded for one specific adhesive among different research institutes worldwide. The main reason for these inconsistent bond-strength measurements is supposedly the current lack of a standard bond-strength testing protocol. This paper (and presentation) aimed to report on an extensive literature review with regard to the different laboratory bond-strength test methods and their data provided, along with a second extensive literature review on clinical effectiveness data of adhesives in terms of retention rates of adhesive Class-V restorations. Combining both systematic reviews, we have subsequently searched for a potential relationship between bond-strength data and clinical outcomes.
1
Dental adhesive technology ANNO 2009
The fast progress in dental adhesive technology has extensively influenced modern restorative dentistry. Although decayed/fractured teeth can be reconstructed minimal-invasively and nearly invisibly using adhesive technology, the clinical longevity of composite restorations is today still too short . Despite the enormous advances made in adhesive technology during the last 50 years, the bonded interface itself remains the Achilles heel of an adhesive filling . Mainly water sorption is thought to destabilize the adhesive–tooth bond, though the actual interfacial degradation mechanisms are far from understood. In this context, several aspects should be considered with regard to the strength and durability of the bond to the two dental hard tissues, enamel and dentin. These include the heterogeneity of tooth structure and composition, the features of the dental surface exposed after cavity preparation, and the characteristics of the adhesive itself, such as its strategy of interaction with both substrates and its basic physicochemical properties. Furthermore, all sorts of chemical and mechanical challenges that are inherent to the oral environment should be taken into account, such as there are moisture, masticatory stresses, changes in temperature and pH, and dietary and chewing related habits .
Modern adhesive approaches can be divided into (1) an etch&rinse, (2) a self-etch (or etch&dry), and (3) nowadays also a self-adhesive approach .
1.1
Etch&rinse
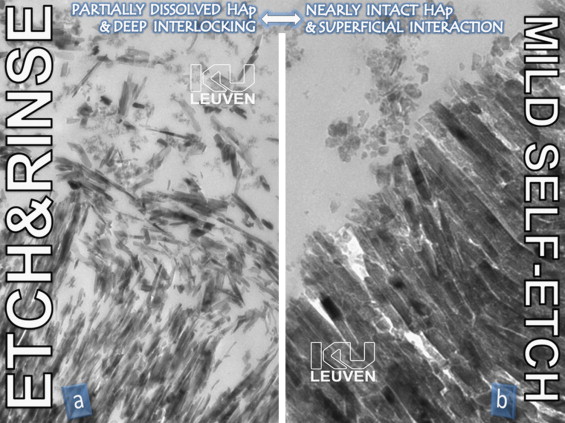
In brief, the multi-step etch&rinse approach involves a phosphoric acid-etch step that at enamel produces deep etch-pits in the hydroxyapatite (HAp)-rich substrate, and at dentin demineralizes up to a depth of a few micrometers to expose a HAp-deprived collagen mesh. The next step involves either a separate priming step followed by the application/curing of a combined primer/adhesive resin following a simplified 2-step procedure, or a separate primer and adhesive resin step following a 3-step procedure. The final objective is to micro-mechanically interlock upon diffusion and in situ polymerization of monomers into the enamel etch-pits ( Fig. 1 a ), the opened dentin tubules and the exposed collagen network, the latter forming the well-documented hybrid layer ( Fig. 2 a ). Without doubt, the micro-mechanical interlocking of tiny resin tags within the acid-etched enamel surface is still today the best achievable bond to enamel . It not only effectively seals the restoration margins on the long term, but also protects the more vulnerable bond to dentin against degradation . On the contrary, etching dentin is a rather aggressive procedure as it dissolves and removes (through rinsing) the natural protection of collagen ( Fig. 2 a), thereby producing a resin–collagen complex that is vulnerable to degradation upon water sorption, possibly enhanced by the documented enzymatic degradation process . As the most intimate and stable intermolecular interaction possible, primary chemical interaction between resin and the mainly organic substance remaining at acid-etched dentin would definitely contribute to the bond durability, but is however lacking . This deficient chemical interaction should most likely be regarded as the major shortcoming of today’s etch&rinse approach. Nevertheless, traditional 3-step etch&rinse adhesives are still today regarded as ‘gold-standard’.

1.2
Self-etch
The self-etch approach can be further subdivided into a ‘strong’ (pH < 1), an ‘intermediately strong’ (pH ≈ 1.5), a ‘mild’ (pH ≈ 2), and an ‘ultra-mild’ (pH ≥ 2.5) self-etch approach depending on the self-etching or demineralization intensity . Self-etching only dissolves the smear layer, but does not remove the dissolved calcium phosphates, as there is no rinse phase. The more intense the self-etching, the more calcium phosphates are dissolved and embedded within the interfacial transition zone . Such resin-encapsulated calcium phosphates within the exposed collagen mesh are however rather soluble ( Fig. 3 ) and may explain the lower laboratory and clinical bonding performance of strong self-etch adhesives, in particular to dentin . At enamel, they however perform in general much better due to this more aggressive self-etching . The less intense the self-etching, the more bur-smear may interfere with the eventual bonding performance ( Fig. 4 ) . In particular ‘mild’ (pH ≈ 2) self-etch adhesives appear to deal reasonably well with bur-smear, producing a submicron hybrid layer with substantial HAp-crystals still protecting the collagen fibrils ( Fig. 2 b). Functional monomers, in particular like 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate), have been proven to interact with this residual HAp through primary ionic binding . The resultant twofold micro-mechanical and chemical bonding mechanism closely resembles that of glass-ionomers ( Fig. 5 ; see below) . However, chemical bonding potential on its own is insufficient; the formed ionic bonds should also be stable in an aqueous environment. Chemical bonding promoted by 10-MDP appeared not only more effective, but also more stable in water than that provided by other functional monomers like 4-MET (4-methacryloyloxyethyl trimellitic acid) and phenyl-P (2-methacryloyloxyethyl phenyl phosphoric acid), in this order . The dissolution rate of the respective calcium salts of these three monomers, as measured by AAS (or atomic absorption spectroscopy), was inversely related to their chemical bonding potential revealed by XPS: the more intense the chemical bonding potential, the less the resultant calcium salt could be dissolved. This finding was further explained in the ‘AD-concept’ or the adhesion–decalcification concept that dictates if molecules will either adhere to or decalcify mineralized tissues .



Two-step self-etch adhesives involve the application of a separate, more hydrophobic adhesive resin after the hydrophilic self-etch primer. This makes the interface more hydrophobic and thus better seals it to the direct benefit of bond durability. Finally, the most simple- and fast-to-use 1-step (self-etch) adhesives generally come with some sacrifice in bonding performance. This lower bonding efficiency has been thoroughly documented in laboratory research, and must be ascribed to, among others, to a lower polymerization conversion and thus inferior mechanical properties, enhanced water sorption through osmosis from the host dentin, potential phase-separation effects when the adhesive solution is low in or free of HEMA (2-hydroxyethyl methacrylate), filler de-bonding within the adhesive resin through hydrolysis of the silane coupling, potential smear interference for ultra-mild self-etch adhesives, and reduced shelf life in particular with regard to one-component formulations .
1.3
Self-adhesive
Glass-ionomers and resin-modified glass-ionomers are ‘self-adhesive’ through submicron hybridization, combined with well-proven primary ionic interaction of polyalkenoic acid with calcium within HAp ( Fig. 5 ). Polyalkenoic acid possesses abundant functional carboxylic groups that ‘grab’ HAp simultaneously at different and remote sites. Other ‘self-adhesive’ materials are the so-called self-adhesive luting composites that have been introduced some years ago . They are often mistakenly termed as ‘self-etching’, while they interact only very superficially with dentin without clear signs of demineralization ( Fig. 6 ). Finally, it is in the line of expectations that such self-adhesive luting composites will soon lead to the development of self-adhesive flowable and later full-restorative composites.





