Fig. 19.1
(a–d) Schematic cascade leading from cell commitment to differentiation in osteoblasts or odontoblasts and the formation of a reparative dentin bridge (asterisk), b bead
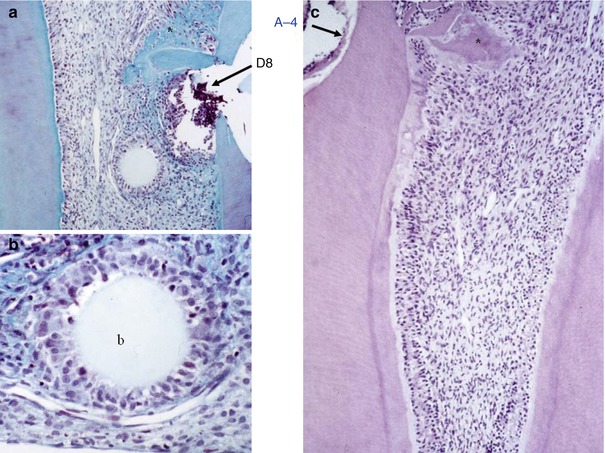
Fig. 19.2
Eight days after the implantation of A-4, pulp cells are committed around agarose beads (a) and start to differentiate into osteo-/odontoblasts (b) (reparative dentin). After 15 days (c), mineralization loci begin to be formed in the crown part (asterisk), whereas in the root reactionary dentin is formed along the root canal lumen behind a calciotraumatic line, b bead
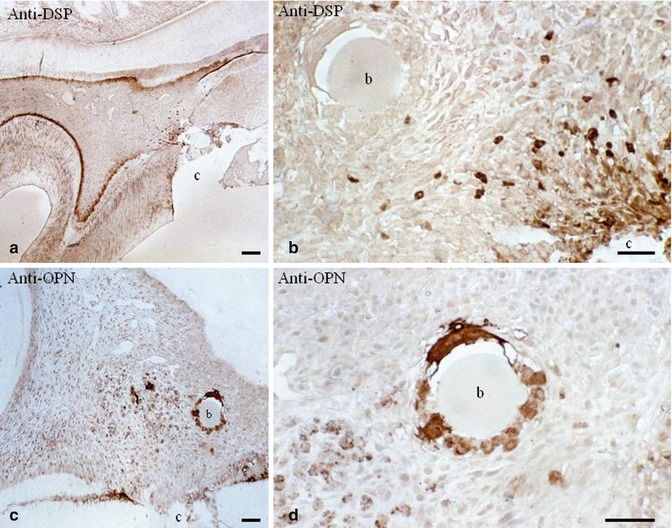
Fig. 19.3
(a, b) Anti-dentin sialoprotein labeling. No labeling is detected around the bead (carrier), but near the pulp exposure DSP-labeled cells are stained positively. They will be implicated in reparative dentin formation. In (c, d), anti-osteopontin labeling is especially dense around the beads (osteoblast labeling), b bead; c cavity
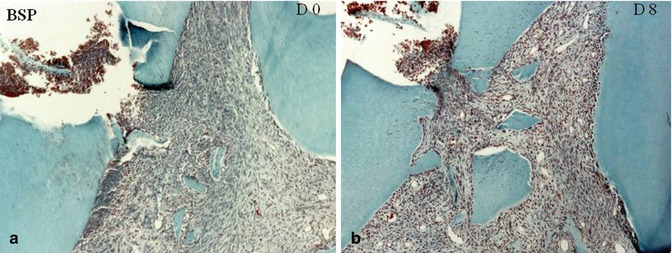
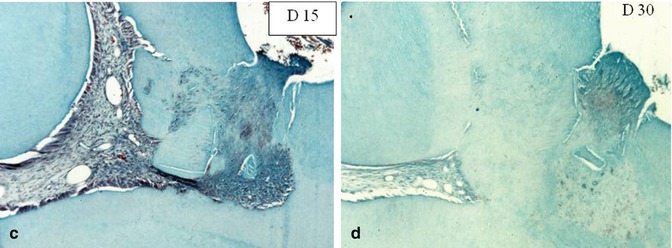
Fig. 19.4
After implantation of bone sialoprotein (BSP), no reaction is detected at day 0–day 8 (a, b). A slight inflammatory process is seen in the pulp horn, due mostly to the dentin debris pushed in the pulp. At day 15 (c), mineralization starts to be formed around dentin debris; and a solid dentin bridge is formed at day 30 (d), occluding homogeneously the pulp exposure
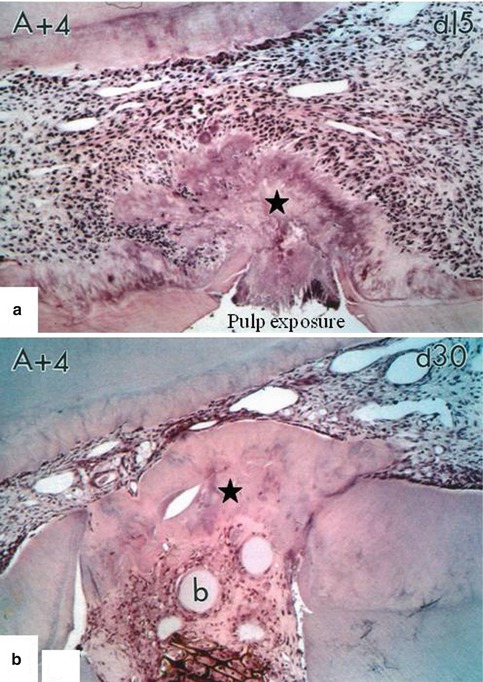
Fig. 19.5
Implantation of A+4 in the pulp. After 15 days (a), the pulp exposure is partially closed by a thin dentin bridge. After 30 days (b), a thick and homogeneous dentin bridge is formed (asterisk). The dental pulp is totally protected from bacterial contamination
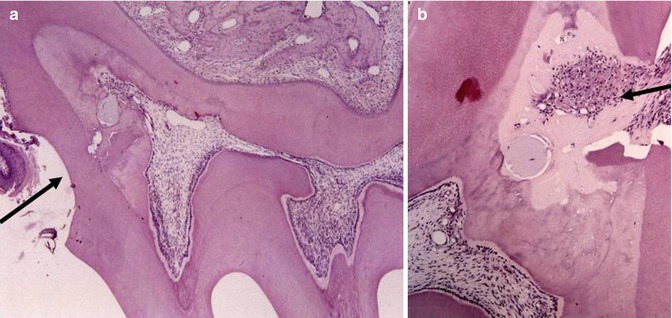
Fig. 19.6
(a) Reactionary dentin (arrow) filling the upper part of the root. (b) Reparative dentin (arrow), closing the pulp exposure. Agarose bead and cell debris are located in the mineralized tissue
Significant progress in the prevention and treatment of pulp and periradicular disease has led to an intensified focus on the ability of the dentin-pulp complex to repair itself and regenerate mineralized tissue. Recent advances in biotechnology and translational research offer hope of providing new treatment modalities that can protect the vital pulp, enable manipulation of reactionary dentinogenesis, and stimulate revascularization of an infected root canal space. Indeed, the volume of the mature pulp is very small (less than 100 μl), so it might seem that regeneration of such a tiny island of tissue could be achieved relatively easily. Unfortunately, this is not the case. The pulp is a highly specialized connective tissue enclosed in a mineralized shell with a limited blood supply and thus poses a unique challenge for the design and development of new therapeutic strategies.
Attempts were made to use dentin extracellular matrix molecules. Rutherford et al. [7, 8] have implanted BMP-7 (OP-1) in the pulp. Osteodentin was formed filling totally the root canal. Better results were obtained with bone sialoprotein (BSP), and a solid homogeneous dentin bridge occluded totally the pulp exposure (Fig. 19.4a–d). The biological effects of a few other ECM molecules were evaluated. This was the case for Dentonin, a 100 amino acid long peptide from MEPE, and for A+4 and A−4, two amelogenin gene splice products. Each molecule contributed to the regeneration of a superficial pulp [9] (Figs. 19.5a, b and 19.6a, b).
It was shown that around the agarose beads used as carrier for bioactive molecules, pulp cells were committed and recruited and they differentiate toward an osteo-/odontogenic lineage. The proliferating cell nuclear antigen (PCNA), a characteristic labeling indicative for cell division, labeled the cell nuclei. Pulp cells migrate, underwent an early differentiation forming a ring around and close to the surface of the carrier agarose beads. They were labeled firstly as mesenchymal cells, then becoming osteopontin positive. Later dentin sialoprotein labeled positively the cells implicated in the formation of a reparative dentin matrix, which further become mineralized. The cascade of differentiation leads to the formation of ortho- or osteodentin (Figs. 19.6a, b and 19.7a, b). These biological approaches allow a better understanding of what is occurring during pulp capping or regeneration.
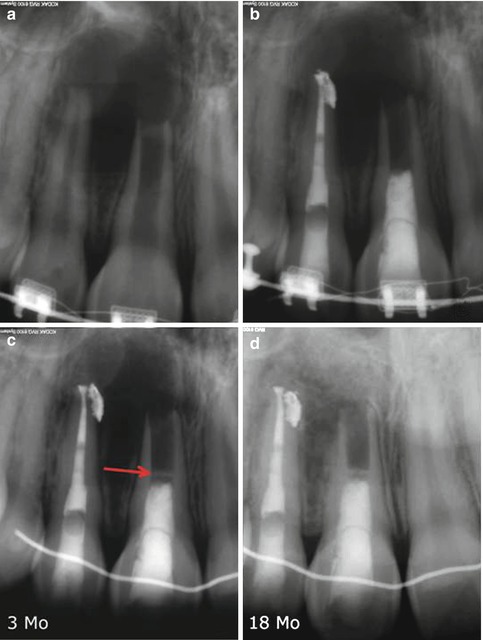

Fig. 19.7
Endodontic treatment by revascularization on tooth #8 on a 16-year-old girl and with conventional approach on tooth #7 (a, b). Note the formation of a mineralized barrier at low distance from coronal filling material (mineral trioxide aggregate) (arrow) at an early stage of healing (3 months postoperative) (c). At an 18-month recall (d), the bone healing is complete and the mineral barrier still present. Nevertheless, no root lengthening, neither apexogenesis, was noticeable
Regenerative endodontics involves two alternatives:
1.
Dentin-pulp complex regeneration (which could also be called dentin-odontoblast complex regeneration), which involves the preservation of pulp vitality and pulp capping
2.
Dental pulp regeneration, which is the regeneration of vital tissue in an infected root canal space
19.2 Dentin-Pulp Complex Regeneration
Clinically, the aims of such treatments are to keep the pulp vital and maintain its homeostatic functions so as to avoid pulpectomy or tooth extraction. Treatment success is based on symptoms reported by the patient as well as the results of relatively rudimentary tests such as thermal tests, electrical tests, tenderness to percussion or palpation, and radiographic assessment. It is well established that clinical signs and symptoms do not correlate with the histological status of the tooth [10]. More advanced assessment using biological research methods allows the investigator to analyze histological structure, cellular behavior, and immunological/inflammation status of the tissue, thus providing more specific indications of pulp responses which may not be evident in clinical studies. To study physiological and reparative processes in the pulp, in vitro experiments can be conducted with immortalized cells or primary cell cultures. However, such studies are limited because they can only mimic biological processes such as mineral production but cannot determine if the tissue produced is actually dentin or another form of mineralized tissue. Nonetheless, these experiments can be supported with RT-PCR or microarray data to identify the likely gene expression profile which induced the mineral production. Confidence in the tissue regeneration results is then enhanced when phenotypic markers associated with dentin production are observed.
19.3 Regeneration/Repair and Remodeling
The bone undergoes constant remodeling, with turnover rates of less than a few months. Thus, gradually, newly secreted bone tissue will merge with older tissue such that the new and old become homogeneous. Some exceptions occur, such as in pseudarthrosis, in which the new nonresident tissue should be considered reparative tissue and is histologically and ultrastructurally distinctly different from that formed through regeneration. Remodeling can also lead to destruction of regenerated tissue (partial or complete) in cases where the tissue is not biologically compatible with its microenvironment [11].
In contrast to the bone, in the tooth, remodeling of dentin does not occur and newly formed tissue will never be replaced. Histologically, tertiary dentin can appear to be similar to secondary dentin; however, it is never truly the same nor continuous with preexisting dentin.
The dentin-pulp interface may be considered a dentin-odontoblast complex, because dentin is uniquely penetrated by odontoblast processes, which form an intimate union with the cohesive and intermingling odontoblast palisade beneath. This layer acts like a membrane, separating itself from the pulp underneath by the acellular layer of Weil. Breakage of this odontoblast membrane by caries, trauma, or iatrogenic damage exposes the pulp tissue, leaving it unprotected and vulnerable.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


