Introduction
Suture expansion stimulates bone growth to correct craniofacial deficiencies but has a high potential of treatment relapse. The objective of this study was to investigate whether there is a dose-dependent relationship between the recombinant human bone morphogenetic protein-2 (rhBMP-2) and bone formation during suture expansion.
Methods
Fifty 6-week-old male New Zealand white rabbits were randomly assigned to 5 groups to receive 0 (control), 0.01, 0.025, 0.1, or 0.4 mg/mL of rhBMP-2 delivered by absorbable collagen sponge placed over the interfrontal suture. The suture was expanded for 33 days by 200 g of constant force via a spring anchored with 2 miniscrew implants. Distance of suture expansion, suture volume, and cross-sectional area after expansion were measured using radiographs with bone markers and microcomputed tomography. Suture widths and mineralization appositional rates were calculated based on the widths between bone labels under an epifluorescent microscope. Software (Multilevel Win 2.0; University of Bristol, Bristol, United Kingdom) was used to model distance of suture expansion over time as polynomials to compare group differences. Wilcoxon signed rank tests were performed to compare the suture volume and cross-sectional area, mineral apposition rate, and suture width between groups. The significance level was set at P = 0.05.
Results
Whereas the sutures were expanded in all groups, sutures were expanded by significantly greater amounts in the control and the 0.01 mg/mL groups without fusing the sutures than in the 0.025, 0.1, and 0.4 mg/mL groups with fusing sutures. Compared with the controls, the 0.01 mg/mL group showed significantly lower suture volumes, cross-sectional areas, and suture widths after expansion. The mineral apposition rate was significantly higher in the 0.01 mg/mL group than in the controls from days 10 to 30.
Conclusions
The 0.01 mg/mL dose of rhBMP-2 delivered by absorbable collagen sponge can stimulate bone formation at the bony edges of the suture during suture expansion; however, higher concentrations cause suture fusion. With an appropriate concentration, rhBMP-2 might facilitate suture expansion for clinical uses.
Suture expansion is a commonly used clinical approach to augment bone in patients with a craniofacial disharmony. Applying mechanical tensile force to widen the suture stimulates new bone formation at the bony edges adjacent to the separated suture; this increases the bone volume. Suture expansion is also called suture distraction osteogenesis because of its similarity to bone distraction osteogenesis but without the osteotomy. Although patient appearance can be significantly improved, the bony edges of the suture tend to shift back into the sutural gap because new bone usually generates at a slower rate than suture expansion. Because of this potential treatment relapse, 3 to 6 months of retention are recommended after suture expansion.
To minimize treatment relapse, optimal approaches for suture expansion should seek to maximize bone formation while expanding the suture. Two possible mechanisms were proposed to control and enhance sutural bone formation. One was to apply an “ideal” mechanical force to expand the suture ; the other was to use pharmaceutical agents to accelerate the bone formation rate during expansion. Using miniscrew implants and controllable force in an animal model, 50 g of continuous force was shown to be more effective in generating sutural bone formation than the same magnitude of intermittent force. The amount of bone formation was proportional to the magnitude of continuous expansion force and its consequent distance of suture expansion. However, the bone formation rate plateaued after exceeding a certain force limit (bone formation rate of 50 g <100 g = 200 g). An increase in sutural width over time with 100 g indicates that the maximum rate of bone formation is slower that the maximum expansion rate. Beyond this limit (100 g of continuous expansion force), greater amounts of force did not increase the bone formation rate, but only increased the sutural gap and subsequent relapse potential. Therefore, an “ideal” mechanical force exists to expand the suture and generate maximal bone formation in a given circumstance.
Alternatively, a pharmaceutical agent, such as recombinant human bone morphogenetic protein-2 (rhBMP-2), can potentially be used to accelerate bone formation during suture expansion. RhBMP-2 is an important osteoinductive protein that enhances bone regeneration and accelerates wound-healing rates in patients with open tibial fractures. Previously, rhBMP-2 delivered by an absorbable collagen sponge has been tested on suture expansion with 100 g of continuous tensile force across the interfrontal suture in rabbits. A concentration of 0.4 mg/mL of rhBMP-2 significantly increased suture bone formation, compared with a lower concentration of 0.1 mg/mL of rhBMP-2. However, administration of rhBMP-2 at 0.1 and 0.4 mg/mL led to closure of the suture after 10 days. This indicates that the stimulating effect of 0.1 mg/mL of rhBMP-2 was still excessive and caused premature suture fusion. Clinically, suture fusion might inhibit further suture growth in adolescents. Therefore, in this study, we proposed to test whether lower concentrations of rhBMP-2 could result in greater expansion of sutures while filling the resultant gaps with bone and preventing suture fusion. A higher continuous expansion force (200 g) was also used. The objective was to investigate whether there is a dose-dependent relationship between rhBMP-2 and bone formation during suture expansion.
Material and methods
The study included 50 male New Zealand white rabbits, 6 weeks of age. The housing, care, and experimental protocol were in accordance with the guidelines of the Institutional Animal Care and Use Committee of Texas A&M University, Baylor College of Dentistry, Dallas.
All animals were anesthetized with ketamine at 75 mg/kg intramuscularly and acepromazine at 5 mg/kg intramuscularly. After skin and periosteum reflection, 2 miniscrew implants (length, 3.0 mm; diameter, 1.7 mm; Dentos, Daegu, Korea) were manually inserted into the bone adjacent to the interfrontal suture. Four tantalum bone markers were tapped into the frontal bone to radiographically quantify suture expansion ( Fig 1 ). The animals were randomly assigned to 5 groups to receive different concentrations of rhBMP-2 (Medtronic, Minneapolis, Minn) delivered by absorbable collagen sponges. The 6 × 8 × 2 mm absorbable collagen sponge was soaked with 0 mg/mL (control), 0.01 mg/mL, 0.025 mg/mL, 0.1 mg/mL, or 0.4 mg/mL of rhBMP-2 according to the manufacturer’s instructions for 15 minutes and placed over the interfrontal suture. After flap closure by 4-0 Vicryl sutures (Ethicon Inc, Somerville, NJ), the suture was expanded using 200 g of constant force applied by a nickel-titanium open-coil spring similar to the procedures in previous studies. Penicillin (27216 IU/kg, intramuscularly) and buprenorphine (0.02 mg/kg, subcutaneously, as needed) were given immediately after surgery.
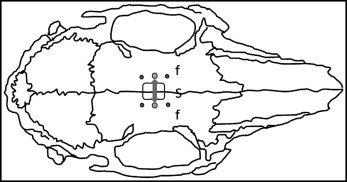
 , tantalum bone markers
, tantalum bone markers  , absorbable collagen sponge
, absorbable collagen sponge  , and direction of expansion force
, and direction of expansion force  .
. To quantify bone formation, calcein (10 mg/kg, intramuscularly; Sigma-Aldrich, St Louis, Mo) and oxytetracycline (6.17 mg/kg, intramuscularly; Merial, Duluth, Ga) were administered to all animals. Calcein was given at days 10 and 30; oxytetracycline was given at day 20. After 33 days of suture expansion, the rabbits were killed with an overdose of beuthanasia (intracardiac injection of 1 cm per animal) and perfused with 70% ethanol.
Animal weights, standardized ventrodorsal cephalometric digital radiographs, and miniscrew implant width measurements were taken under anesthesia at 10, 20, and 30 days. The rabbit head was oriented parallel to the stage, and a metal scale was tapped on the film for measurement calibration. The digital radiographic images were taken with the cone positioned perpendicular to the film with a fixed distance between the cone and the film. Caliper width measurements between each miniscrew implant pair were taken at the outermost margins immediately above the guide wire. The width of the bone markers was calculated as the average of distances for anterior and posterior paired bone markers visualized on the radiographs using Visix software (Air Techniques, Melville, NY). After 2 weeks, the radiographs were remeasured for evaluating the intraexaminer measurement error (average distance between anterior bone markers, 0.098 mm; average distance between each miniscrew implant pair, 0.118 mm; average distance between posterior bone markers, 0.089 mm) with Dahlberg’s method (√∑d2/2n).
A standardized region, including the interfrontal suture and neighboring bone, was harvested and fixed with 70% ethanol for 2 weeks without decalcification.
The specimens were placed in a clear scanning tube, and the interfrontal suture was aligned along the tube axis and scanned using microcomputed tomography (μCT35; Scanco Medical, Basserdorf, Switzerland) at 55 kVp and 72 mA. One hundred serial scanned slices with a slice thickness of 7-μm resolution from the region between the miniscrew implants were selected for each specimen and reconstructed with the Scanco software. For the nonfused sutures, the 3-dimensional suture volume of the remaining gap after expansion and the cranial bone thickness were measured, and the cross-sectional area was calculated as suture volume divided by cranial bone thickness ( Fig 2 ).
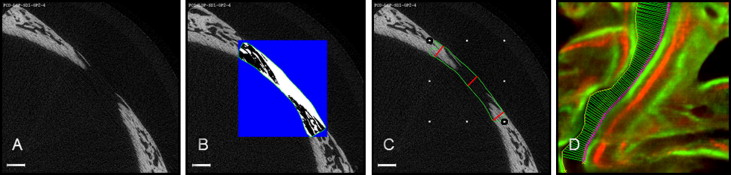
After the microcomputed tomography analysis, each specimen was dehydrated with an ascending series of ethanol, embedded in methyl methacrylate, and sectioned (approximately 100-μm thick sections) coronally with an Isomet low-speed diamond saw (Buehler, Lake Bluff, Ill) (3 sequential sections per animal), followed by grinding and polishing. An epifluorescence microscope (80i; Nikon, Brighton, Mich) (excitation wave lengths of 390 nm for oxytetracycline and 485 nm for calcein) with a camera (Coolsnap K4; Photometrics, Tucson, Ariz) were used to capture fluorescent images of each histologic section. Using Osteo II software (Bioquant Image Analysis, Nashville, Tenn), a blinded examiner (J.S.) traced the fluorescent bone labels, and interlabel distances were automatically measured by the Bioquant software. The mineral appositional rate was calculated as the average interlabel distance (μm) between the 10-day and 30-day labels divided by 20 days. Suture width of the remaining gap after expansion was measured and averaged as the interlabel width between the 30-day labels at the bony edges of the suture ( Fig 2 , D ).
Statistical analysis
Statistical analyses were performed using software (Multilevel Win 2.0; University of Bristol, Bristol, United Kingdom), and 95% confidence intervals ( P <0.05) were obtained. The curves describing the changes of the miniscrew implant widths, radiographic miniscrew implant widths, and weight measurements were modeled over time as polynomials. The fixed part of the models described changes as a function of time and statistically compared the groups. The random part of the models had animals at the higher level and their repeated measures at the lower level, nested in the higher level. Iterative generalized least squares were used to estimate the polynomials. To statistically evaluate differences at the end of the experiment, the constant of each polynomial was fixed at day 30. Wilcoxon signed rank tests were performed to compare suture volume, cross-sectional area, mineral appositional rate, and suture width between groups using SPSS software (version 15.0; SPSS, Chicago, Ill).
Results
No obvious signs of discomfort or infection surrounding the miniscrew implants were noticed in any animal. The overall miniscrew implant success rate was 90% (45 of 50). Five pairs of miniscrew implants loosened and were removed during expansion: 2 pairs in the control group, 1 pair in the 0.01 mg/mL group, and 2 pairs in the 0.4 mg/mL group before day 10. Measurements of these animals were treated as missing.
Widths between both miniscrew implants and tantalum bone markers increased significantly in all 5 groups after expansion ( Fig 3 ). Although changes in miniscrew implant width over time were slightly greater than were the changes in bone marker width, both miniscrew implant and bone marker width changes over time were similar to each other in each group. Bone marker width, indicating distance of suture expansion, increased significantly more ( P <0.05) in the control (12.2 ± 1.23 mm) and the 0.01 mg/mL (11.7 ± 2.24 mm) groups than in the 0.025 mg/mL (6.5 ± 2.41 mm), 0.1 mg/mL (5.5 ± 1.67 mm), and 0.4 mg/mL (5.3 ± 2.31 mm) groups. There was no difference between the control and the 0.01 mg/mL rhBMP-2 groups. For the other concentrations, no difference was found with the control group for the first 10 days; afterward, there was no further significant increase of the distances.




