The pulp-dentin complex is a strategic and dynamic barrier to various insults that plague the dentition. Researchers have yet to understand the complete potential of this shifting junction and its components. The most common cause of injury to the pulp-dentin complex is carious breakdown of enamel and dentin. In recent years, there has been a change in restorative management of caries. The emphasis is on strategies to preserve dentin and protect the pulp. This article provides a brief review of the effect of caries on the pulp, of subsequent events on the periradicular tissues, and of current understanding of treatment modalities.
- •
The pulp-dentin complex is a strategic and dynamic barrier to various insults that plague the dentition. Researchers have yet to understand the complete potential of this constantly shifting junction and its components, the predentin, dentinal tubules, odontoblast layer; their processes; and the vascular and neural elements.
- •
The most common cause of injury to the pulp-dentin complex is the carious breakdown of the enamel and dentin, leading to pathologic changes in the pulp and periradicular area.
- •
In recent years, there has been a change in the restorative management of caries. Classically, complete removal of caries was a basic principle strictly taught for many years in dental schools. The current emphasis, however, is on strategies to preserve dentin and protect the pulp, sometimes with incomplete removal of caries.
Introduction
The dentin and pulp function physiologically as a single unit, the pulp-dentin complex. This complex is a dynamic tissue that responds to mechanical, bacterial, or chemical irritation in several ways to decrease that irritation. The vitality and dentin repair potential of the pulp are dependent on the survival of the odontoblasts beneath the site of injury. Apart from dentinogenesis, odontoblasts also play important roles as defense cells and as thermal and mechanical sensory receptors. Thus, the net effect of caries or a restorative procedure on the pulp is the result of a complex interaction of many factors. These factors include the thickness and permeability of the intervening dentin, the health of the underlying pulp, mechanical injury to odontoblast processes during cavity preparation, the possible toxicity of the restorative material, and microbial leakage.
Although caries are the principal reason for placement of initial restorations, it is important to discriminate between pulp management of a carious insult and the events that can affect the pulp in the absence of caries. The latter include injurious events that occur after deep cavity preparation or bacterial leakage from restorations. This article summarizes current understanding of the management of carious insults to the pulp and the subsequent effects on the periradicular tissues as they relate to current views on tissue regeneration.
Pulp response to caries
Contrary to most connective tissues, the dental pulp does not tolerate injury easily and is more vulnerable for 3 reasons: (1) it is a large volume of tissue with a small volume of blood supply; (2) it is a terminal circulation with few, if any, collateral vessels; and (3) it is confined in calcified tissue walls. As a result, early caries lesions produce cytoplasmic changes in the odontoblasts that are evident at the ultrastructural level. Before an active lesion reaches the dentinoenamel junction, a significant reduction in the cytoplasm-to-nucleus ratio of odontoblasts and a concomitant reduction in predentin thickness have been observed. The dynamics of caries progression cannot be explained solely on a chemical basis and are influenced by interaction between the metabolic activities of the bacterial biofilms and the response from odontoblasts. Generation of microbial metabolic products and matrix degradation products and the release of growth factors from the dentin extracellular matrix influence disease progression. At the same time, the tubular characteristics of dentin and the extent of tubular sclerosis derived from the reactionary response of the pulp-dentin complex affect the permeability of dentin. Due to this variability in caries progression, there is no single response to the disease. Rather, the pulp-dentin complex exhibits a broad spectrum of responses that represents a summation of injury, defense, and repair events. The relative contributions and interactions of these interrelated responses are critical in determining the fate of the pulp-dentin complex and its ability to survive the caries assault. Although discussion of these interactions and their is beyond the scope of this article, a few examples provide a brief overview.
A positive hydrostatic pressure from the pulpal circulation results in outward fluid flow when tubules are exposed. Outward fluid flow is a transudate of plasma and may contribute to pulpal protection because it contains proteins (immunoglobulins, albumin, and fibrinogen) and minerals (calcium and phosphates). Outward fluid flow also limits the rate of diffusion of noxious agents in a pulpal direction. To initiate injury, bacterial acids, soluble plaque metabolic products, and cell wall components have to diffuse pulpward against an outward flow of dentinal fluid. Although outward fluid flow reduces the rates of permeation of microbial and chemical solutes, endotoxins derived from the lipopolysaccharides may be observed in vital pulps of human carious teeth. Alternatively, the buffering action of dentin (0.6 mm or thicker) can effectively buffer bacterial acids found in carious dentin, such as lactic and acetic acids, and avoid direct injury to the pulp. Taken together, these findings may explain why injury responses to the odontoblasts and subodontoblastic cells are apparent in active carious lesions that involve more than a quarter of the thickness of enamel.
In response to the carious insult, the pulp-dentin complex initiates both innate and adaptive immune response. Innate immunity plays an important role in shallow caries after the initial enamel caries reaches the dentinoenamel junction. During this initial stage, pulpal responses are likely low grade and chronic. The transition from innate to adaptive immunity probably occurs in irreversibly inflamed pulps that are separated by less than 2 mm of deep carious dentin. This transition may also be influenced by repair reactions involving dentinal sclerosis and tertiary dentinogenesis, which modify the permeability of the dentin matrix.
When the pulp is subjected to a gradually progressive insult, a major part of the pulp’s response is the deposition of minerals within the dentinal tubules, occluding the tubules against further ingress of noxious stimuli. Caries lesions may progress slowly or rapidly or become arrested. Consequently, sclerosis of the dentinal tubules is either absent or minimal in rapidly progressing active lesions. In the absence of complete occlusion or with minimal tubular occlusion, rapid diffusion of the metabolic and degradation products could overwhelm the pulp’s defensive responses and result in pulpal inflammation, absence of tertiary dentinogenesis, and severe pulpal injury. Conversely, a transparent zone of sclerotic dentin observed at the base of the caries-affected dentin can reduce dentin permeability and impede the diffusion of bacterial products or solubilized matrix components along the tubules, thereby delaying lesion progression. Because the thickness of this zone is higher in slow progressing and arrested lesions, this form of defense and healing of pulp tissue are more favorable in response to slowly progressing or arrested lesions.
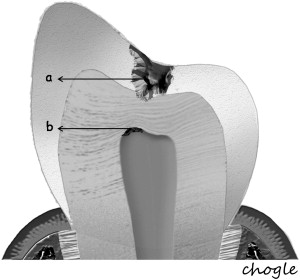
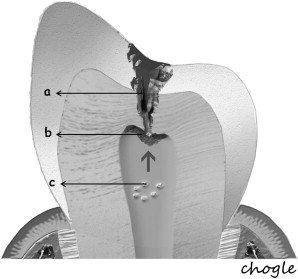
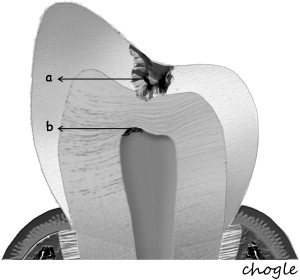
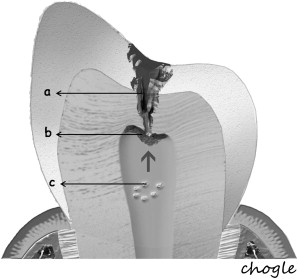
Additionally, the pulp-dentin complex may react to stimuli with tertiary dentin formation. Unlike primary and secondary dentinogenesis, tertiary dentinogenesis is a focused reaction in the vicinity of the dentin that is directly affected by the caries process. Recent reports have redefined tertiary dentinogenesis in relation to the nature of the injury. The term, reactionary dentinogenesis , has been adopted to describe the secretion of a tertiary dentin matrix by primary odontoblasts that have survived injury to the tooth ( Fig. 1 ). This is a wound healing reaction to produce circumpulpal dentin in response to slowly progressing dentinal caries. Conversely, reparative dentinogenesis refers to the secretion of tertiary dentin after the death of the primary odontoblasts underlying the injury, after the differentiation of odontoblast-like cells ( Fig. 2 ). Reparative dentin formation occurs in response to deep dentinal caries and represents a more complex sequence of biologic events compared to reactionary dentinogenesis, including progenitor cell recruitment and differentiation. When the pulp is exposed in advanced lesions, reparative dentinogenesis results in dentin bridge formation, which restores the functional integrity of the pulp-dentin complex. In actively progressing advanced lesions involving pulpal exposure, however, the inflammatory reactions may become acute and uncontrolled as bacteria approach and penetrate the pulp. Although inflammation is regarded as a defense response, severe reactions can result from continued ingress of bacteria, producing irreversible destruction of the pulp that eventually results in pulpal necrosis and development of periradicular lesions.
Pulp response to caries
Contrary to most connective tissues, the dental pulp does not tolerate injury easily and is more vulnerable for 3 reasons: (1) it is a large volume of tissue with a small volume of blood supply; (2) it is a terminal circulation with few, if any, collateral vessels; and (3) it is confined in calcified tissue walls. As a result, early caries lesions produce cytoplasmic changes in the odontoblasts that are evident at the ultrastructural level. Before an active lesion reaches the dentinoenamel junction, a significant reduction in the cytoplasm-to-nucleus ratio of odontoblasts and a concomitant reduction in predentin thickness have been observed. The dynamics of caries progression cannot be explained solely on a chemical basis and are influenced by interaction between the metabolic activities of the bacterial biofilms and the response from odontoblasts. Generation of microbial metabolic products and matrix degradation products and the release of growth factors from the dentin extracellular matrix influence disease progression. At the same time, the tubular characteristics of dentin and the extent of tubular sclerosis derived from the reactionary response of the pulp-dentin complex affect the permeability of dentin. Due to this variability in caries progression, there is no single response to the disease. Rather, the pulp-dentin complex exhibits a broad spectrum of responses that represents a summation of injury, defense, and repair events. The relative contributions and interactions of these interrelated responses are critical in determining the fate of the pulp-dentin complex and its ability to survive the caries assault. Although discussion of these interactions and their is beyond the scope of this article, a few examples provide a brief overview.
A positive hydrostatic pressure from the pulpal circulation results in outward fluid flow when tubules are exposed. Outward fluid flow is a transudate of plasma and may contribute to pulpal protection because it contains proteins (immunoglobulins, albumin, and fibrinogen) and minerals (calcium and phosphates). Outward fluid flow also limits the rate of diffusion of noxious agents in a pulpal direction. To initiate injury, bacterial acids, soluble plaque metabolic products, and cell wall components have to diffuse pulpward against an outward flow of dentinal fluid. Although outward fluid flow reduces the rates of permeation of microbial and chemical solutes, endotoxins derived from the lipopolysaccharides may be observed in vital pulps of human carious teeth. Alternatively, the buffering action of dentin (0.6 mm or thicker) can effectively buffer bacterial acids found in carious dentin, such as lactic and acetic acids, and avoid direct injury to the pulp. Taken together, these findings may explain why injury responses to the odontoblasts and subodontoblastic cells are apparent in active carious lesions that involve more than a quarter of the thickness of enamel.
In response to the carious insult, the pulp-dentin complex initiates both innate and adaptive immune response. Innate immunity plays an important role in shallow caries after the initial enamel caries reaches the dentinoenamel junction. During this initial stage, pulpal responses are likely low grade and chronic. The transition from innate to adaptive immunity probably occurs in irreversibly inflamed pulps that are separated by less than 2 mm of deep carious dentin. This transition may also be influenced by repair reactions involving dentinal sclerosis and tertiary dentinogenesis, which modify the permeability of the dentin matrix.
When the pulp is subjected to a gradually progressive insult, a major part of the pulp’s response is the deposition of minerals within the dentinal tubules, occluding the tubules against further ingress of noxious stimuli. Caries lesions may progress slowly or rapidly or become arrested. Consequently, sclerosis of the dentinal tubules is either absent or minimal in rapidly progressing active lesions. In the absence of complete occlusion or with minimal tubular occlusion, rapid diffusion of the metabolic and degradation products could overwhelm the pulp’s defensive responses and result in pulpal inflammation, absence of tertiary dentinogenesis, and severe pulpal injury. Conversely, a transparent zone of sclerotic dentin observed at the base of the caries-affected dentin can reduce dentin permeability and impede the diffusion of bacterial products or solubilized matrix components along the tubules, thereby delaying lesion progression. Because the thickness of this zone is higher in slow progressing and arrested lesions, this form of defense and healing of pulp tissue are more favorable in response to slowly progressing or arrested lesions.
Additionally, the pulp-dentin complex may react to stimuli with tertiary dentin formation. Unlike primary and secondary dentinogenesis, tertiary dentinogenesis is a focused reaction in the vicinity of the dentin that is directly affected by the caries process. Recent reports have redefined tertiary dentinogenesis in relation to the nature of the injury. The term, reactionary dentinogenesis , has been adopted to describe the secretion of a tertiary dentin matrix by primary odontoblasts that have survived injury to the tooth ( Fig. 1 ). This is a wound healing reaction to produce circumpulpal dentin in response to slowly progressing dentinal caries. Conversely, reparative dentinogenesis refers to the secretion of tertiary dentin after the death of the primary odontoblasts underlying the injury, after the differentiation of odontoblast-like cells ( Fig. 2 ). Reparative dentin formation occurs in response to deep dentinal caries and represents a more complex sequence of biologic events compared to reactionary dentinogenesis, including progenitor cell recruitment and differentiation. When the pulp is exposed in advanced lesions, reparative dentinogenesis results in dentin bridge formation, which restores the functional integrity of the pulp-dentin complex. In actively progressing advanced lesions involving pulpal exposure, however, the inflammatory reactions may become acute and uncontrolled as bacteria approach and penetrate the pulp. Although inflammation is regarded as a defense response, severe reactions can result from continued ingress of bacteria, producing irreversible destruction of the pulp that eventually results in pulpal necrosis and development of periradicular lesions.
Vital pulp therapy
Vital pulp therapy is aimed at sealing the pulp after injury and stimulating the formation of tertiary dentin. This can be achieved through direct and indirect pulp capping, pulpotomy, and other therapies that protect the pulp from the chemical, bacterial, mechanical, and thermal insults due to attrition, erosion, caries, restoration procedures, and restoration placement. The dental pulp, when exposed, may respond favorably to application of a variety of materials used in pulp capping procedures. Many studies have confirmed the formation of hard tissue over the site of the exposure. This may demonstrate that the dental pulp has an intrinsic capacity to heal. The clinical outcomes differ, however, in their inferences as to the predictability of hard tissue formation. The factors affecting the outcome of pulpal capping procedures may be categorized broadly as those related to the pulp exposure and the material used to seal the exposure.
Indirect Pulp Treatment
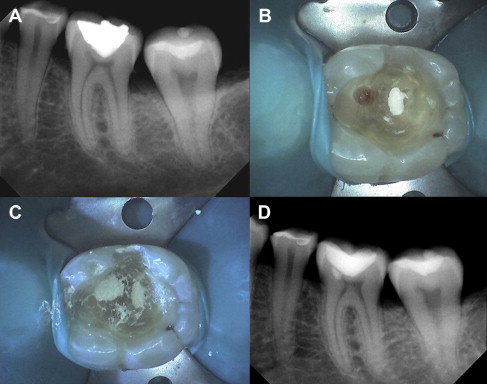
When the bacterial penetration reaches less than a 0.75 mm away from the pulp, the degree of pulpal disease becomes extreme. In other words, the pulp remains reasonably intact if there is at least 0.75 mm or more of intact, bacteria-free dentin between the caries lesion and the pulp. This may be due to the increase in number of tubules per unit area, the tubule diameter increase closer to the pulp, and the ability of the bacterial by-products (enzymes, toxins, and so forth) to penetrate the remaining tubular distance causing pulpitis. When the majority of the caries are removed, except for the deepest layer overlying some intact dentin, then the bulk of the lactic acid–producing complex is eliminated ( Fig. 3 ). Additionally, it has been hypothesized that if the source of nutrition for the cariogenic bacteria is eliminated, the organisms would die, resulting in an arrested carious lesion.
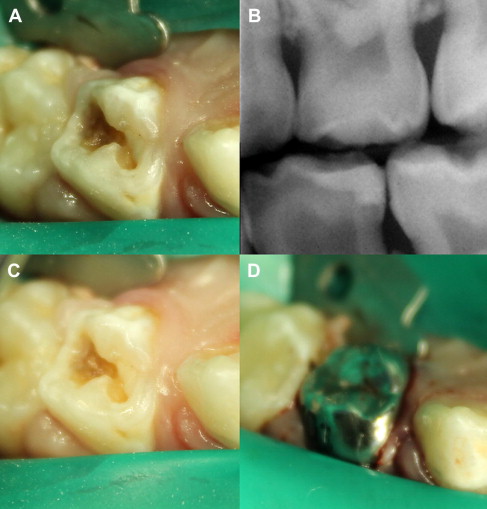
In an indirect pulp treatment procedure, stepwise excavation of caries lesions in the permanent dentition involves 2 main steps. The initial removal of gross caries and subsequent placement of a material, in an attempt to deprive bacteria of a substrate, prevent a direct carious pulpal exposure, and remineralize the remaining caries lesion, with a subsequent return to normal tissue pH. In vitro studies of severely carious teeth, however, found that clinical observations of dentin color changes and mineral increases in the remaining carious dentin do not always represent a change in the bacterial content. Although the microbiologic bioburden may be reduced, it is still present. In a clinical setting, retaining a layer of carious dentin (indirect procedures) presents the dilemma most clinicians face in deciding how to treat these lesions. Continued research and clinical trials are needed to develop the appropriate case selection guidelines, treatment approaches, and materials needed to maximize clinical success.
Pulp Exposure
The classic studies of Kakehashi and colleagues demonstrate bacterial infection as a critical etiologic factor for pulpal necrosis. The extent of damage from microbial contamination may vary based on the size and chronicity of the exposure, pulp status, and material used to seal the exposure.
Several studies suggest that the size of the pulpal exposure may influence case selection because large pulpal exposures may have greater risk of microleakage and be difficult to restore. ppartial pulpotomies after traumatic crown fractures, however, have demonstrated a 96% success rate with close to 3-year follow-up, including pulpal exposures ranging from 0.5 mm to 4.0 mm. Thus, the size of the exposure may not play a major role, at least within this range.
The duration of pulpal contamination, although important, remains a controversial factor in terms of successful pulp capping. Many clinicians believe that longer periods of contamination by oral microorganisms and debris reduce the chance of success. This is supported by results from animal studies that indicate that the success of Ca(OH) 2 pulp capping is reduced from 93% to 56% when microbial contamination is extended from 1 hour to 7 days. Alternatively, clinical studies in younger patients with up to 3 months of pulp exposure demonstrate a 93% radiographic success rate for partial pulpotomy at a mean follow-up of 4.5 years. As a result, the superficial pulp in younger patients seems more resistant to bacterial invasion than the mature pulp in older patients. Furthermore, the size of the pulp chamber and root canal systems of younger patients mitigates toward a larger volume of pulpal tissue, hence greater success in younger patients.
The inflammatory response of the pulp to bacteria and their by-products and the trauma of caries removal may increase the amount of bleeding of exposed dental pulp tissue. This can adversely affect the effective seal against bacterial invasion and lead to development of a chronic inflammatory infiltrate and inhibition of tertiary dentin formation. Therefore, the use of a hemostatic agent may be useful in vital pulp procedures to clot the capillaries within the subjacent pulp tissue. Several studies have examined the use of hemostatic agents placed over the exposure to halt hemorrhage with conflicting results, such as sodium hypochlorite, ferric sulfate, and chlorhexidine digluconate. Further work should better define the use of these agents, especially when used in combination with other materials, such as mineral trioxide aggregate (MTA), that is now suggested for these procedures.
Materials of Pulp Capping
For a successful outcome in vital pulp therapy, the healing response must demonstrate rapid hard tissue formation at the pulp-material interface with minimal inflammatory response. This desirable healing process should occur when any substance is applied directly to the pulpal exposure site that is capable of stimulating dentinogenesis. One study using a cell culture model system reported that calcium hydroxide (Ca[OH] 2 )inhibited macrophage function and reduced inflammatory reactions when used in direct pulp capping and pulpotomy procedures. In another study, an adhesive system applied to exposed human pulp tissue caused large areas of neutrophil infiltration and death of odontoblasts, thereby inhibiting pulp repair. Together, these studies suggest that the sealing ability of the agent, the method of placement (eg, minimizing the impaction of pulp capping agents in dental pulp), and the chemical nature of the pulp capping material are all critical factors in desirable pulpal healing.
Calcium hydroxide
The introduction of Ca(OH) 2 , from a historical perspective, played an important role in the development of vital pulp therapy. Recently, it has been shown that calcium ions released from Ca(OH) 2 stimulate fibronectin synthesis by dental pulp cells, which in turn may induce the differentiation of pulp progenitor cells into mineralized tissue-producing phenotypes. Cross-sections of pulps treated for more than 6 weeks demonstrated a superior amorphous layer of tissue debris and Ca(OH) 2 , a middle layer of a coarse meshwork of fibers identified as fibrodentin, and an inner layer showing tubular osteodentin. Apart from the ability to form a dentin bridge in the subjacent pulp tissue, Ca(OH) 2 has demonstrated additional benefits, such as antimicrobial characteristics. In a primate study with a 1-year to 2-year follow-up, Ca(OH) 2 -induced dentin bridge formation occurred in 78 of 91 (85%) exposed and contaminated dental pulps, whereas 10% of the pulps in the study sample became necrotic. Despite the successful use of Ca(OH) 2 as a pulp capping agent for 60 years, predictable outcomes remain a problem. For example, a retrospective study that examined Ca(OH) 2 pulp capping of carious exposures in 123 teeth, revealed that 45% failed in the 5-year group and 80% failed in the 10-year group. Additionally a summary of several primate studies involving direct pulp capping with Ca(OH) 2 reported several inflamed and infected pulps after a follow-up period of 1 years to 2 years. The investigators questioned the long-term efficacy of commercially available Ca(OH) 2 bases, particularly in light of the potential for microleakage. This has led to newer studies comparing it with other materials. One such material, MTA, has generated great interest for direct pulp capping and vital pulp therapy.
Mineral trioxide aggregate
MTA is the material of choice for correcting procedural errors as well as for root-end filling material in apicoectomy procedures. In both instances, the material has been shown e tissue compatible, encouraging the formation of new cementum-like hard tissue with restoration of the periodontal ligament, and is considered to have significant osteogenic potential. MTA is currently the alternative material for Ca(OH) 2 in direct pulp capping procedures ( Fig. 4 ). MTA was compared with Ca(OH) 2 in young permanent teeth undergoing apexogenesis (coronal pulpotomy, retention of root system vital pulp tissue, and immature root formation). Two of 14 teeth in the Ca(OH) 2 group failed because of pain and swelling, whereas all in the MTA group seemed successfully treated. When MTA and Ca(OH) 2 were compared in direct pulp capping procedures in dog teeth, MTA presented a higher success rate than Ca(OH) 2 , with a lower occurrence of infection and pulpal necrosis. A more recent randomized clinical trial compared the pulpal responses with iatrogenic pulpotomy performed in healthy human teeth using MTA or Dycal. Pulpal wounds treated with MTA were mostly free from inflammation after 1 week and covered with a compact hard tissue barrier within 3 months whereas teeth treated with Dycal revealed distinctly less consistent formation of a hard tissue barrier and presence of pulpal inflammation at up to 3 months. Collectively, the results of all these studies indicate that MTA is as successful as or more successful than Ca(OH) 2 in vital pulp therapy procedures.