Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Identify etiologic factors causing pulp inflammation.
- 2.
Explain the mechanism of spread of inflammation in the pulp.
- 3.
Explain why it is difficult for the pulp to recover from severe injury.
- 4.
List specific and nonspecific mediators of pulpal inflammation.
- 5.
Classify pulpal diseases and their clinical and histologic features.
- 6.
Describe the mechanisms and explain the consequences of the spread of pulpal inflammation into periradicular tissues and the subsequent inflammatory and immunologic responses.
- 7.
Classify periradicular lesions of pulpal origin.
- 8.
Identify and distinguish between histologic features and clinical signs and symptoms of acute apical periodontitis, chronic apical periodontitis, acute and chronic apical abscesses (suppurative apical periodontitis), and condensing osteitis.
- 9.
Describe the steps involved in repair of periradicular pathosis after successful root canal treatment.
- 10.
Identify and describe, in general, nonendodontic pathologic lesions that may mimic endodontic periradicular pathosis.
Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Identify etiologic factors causing pulp inflammation.
- 2.
Explain the mechanism of spread of inflammation in the pulp.
- 3.
Explain why it is difficult for the pulp to recover from severe injury.
- 4.
List specific and nonspecific mediators of pulpal inflammation.
- 5.
Classify pulpal diseases and their clinical and histologic features.
- 6.
Describe the mechanisms and explain the consequences of the spread of pulpal inflammation into periradicular tissues and the subsequent inflammatory and immunologic responses.
- 7.
Classify periradicular lesions of pulpal origin.
- 8.
Identify and distinguish between histologic features and clinical signs and symptoms of acute apical periodontitis, chronic apical periodontitis, acute and chronic apical abscesses (suppurative apical periodontitis), and condensing osteitis.
- 9.
Describe the steps involved in repair of periradicular pathosis after successful root canal treatment.
- 10.
Identify and describe, in general, nonendodontic pathologic lesions that may mimic endodontic periradicular pathosis.
Irritants
Irritation of pulpal or periradicular tissues can result in inflammation. These irritants can be broadly classified as nonliving or living. Nonliving causes of inflammation may be mechanical, thermal, or chemical. Viable irritants include microorganisms and viruses.
Mechanical Irritants
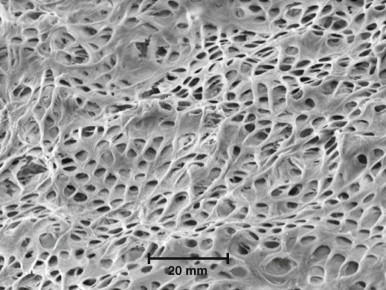
Mechanical irritants, such as deep cavity preparations, removal of tooth structure without proper cooling, impact trauma, occlusal trauma, deep periodontal curettage, and orthodontic movement of teeth may lead to alterations in the underlying pulp. Transient changes, such as aspiration of odontoblasts into the dentinal tubules, are usually reversible in healthy pulps ( Fig. 4.1 ). In typical clinical situations, however, the pulpal tissue is already inflamed due to the presence of caries or previous restorative procedures. If proper precautions are not taken, cavity or crown preparations may damage subjacent odontoblasts. The number of tubules per unit of surface area and the tubules’ diameter increase closer to the pulp ( Fig. 4.2 ). As a result, dentinal permeability is greater closer to the pulp than near the dentinoenamel junction (DEJ) or cementodentinal junction (CDJ). Therefore the potential for pulp irritation increases as more dentin is removed (i.e., as cavity preparation deepens and reaches closer to the pulp). Pulp damage is roughly proportional to the amount of tooth structure removed, in addition to the depth of removal. Also, operative procedures without water coolant cause more irritation than those performed under water spray. A study of the reactions and vascular changes occurring in experimentally induced acute and chronic pulpitis demonstrated increased permeability and dilation of blood vessels in the early stages of pulpitis. Investigations in rodent models designed to determine the impact of heat generation on the dental pulp have shown that elevation of pulpal temperature above 42°C up-regulate heat shock proteins (HSPs). HSP-70 plays a protective role, and its levels return to baseline within a few hours after removal of the heat stimulus.
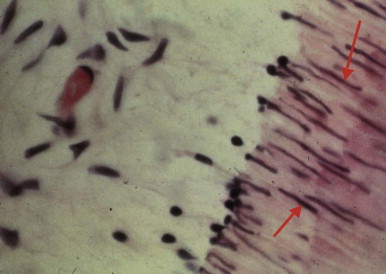
Impact injuries with or without crown or root fractures may cause pulpal damage (see Chapter 11 ). The severity of trauma and degree of apical closure of the root are important factors in the pulp’s ability to recover. Teeth undergoing mild to moderate trauma and those with immature apices have a better chance of pulpal survival compared with those suffering severe injury or those with closed apices. Application of forces beyond the physiologic tolerance of the periodontal ligament (PDL) during orthodontic tooth movement results in disturbance of the blood and nerve supply of the pulp tissue. The resulting changes include atrophy of cells and alteration of nerve axons. In addition, orthodontic movement may initiate resorption of the apex, usually without a change in vitality. Deep scaling and curettage may injure apical vessels and nerves, resulting in pulpal damage (see Chapter 7 ).
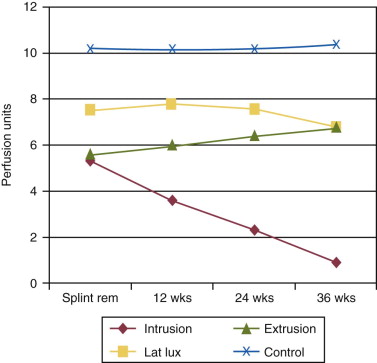
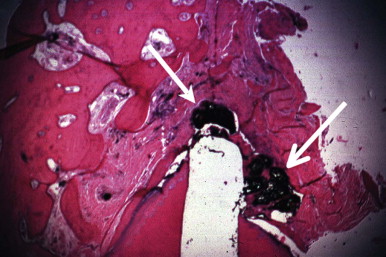
Periradicular tissues can be mechanically irritated and inflamed by impact trauma, hyperocclusion, endodontic procedures and accidents, pulp extirpation, overinstrumentation of root canals, perforation of the root, and overextension of the root canal filling materials. Intrusion injuries are more likely to lead to pulp necrosis than are lateral or extrusion injuries ( Fig. 4.3 ). Mechanical irritation by instruments may occur during canal preparation. Inaccurate determination of canal length is usually the cause of overinstrumentation and the subsequent inflammation. In addition, lack of an adequate apical resistance form created during cleaning and shaping can cause overextension of filling materials into the periapical tissues, causing physical and chemical damage ( Fig. 4.4 ).
Chemical Irritants
Chemical irritants of the pulp include various dentin cleansing, sterilizing, and desensitizing substances, in addition to some of the substances present in temporary and permanent restorative materials and cavity liners. Antibacterial agents, such as silver nitrate, phenol with and without camphor, and eugenol, have been used in an attempt to “sterilize” dentin after cavity preparations. The effectiveness of many of these products as dentin sterilizers is questionable, and their cytotoxicity can cause inflammatory changes in the underlying dental pulp. Other irritating agents include cavity cleansers, such as alcohol, chloroform, hydrogen peroxide, and various acids; chemicals present in desensitizers; cavity liners and bases; and temporary and permanent restorative materials.
Antibacterial irrigants used during cleaning and shaping of root canals, intracanal medications, and some compounds present in obturating materials are examples of potential chemical irritants of periradicular tissues. Most irrigants and medicaments are toxic and are not biocompatible. Trevino and associates tested the impact of 17% ethylenediaminetetraacetic acid (EDTA) alone or in combination with sodium hypochlorite (NaOCl) or chlorhexidine on the viability of stem cells isolated from the apical papilla. Their results showed that EDTA was best at supporting cell viability (89% cell survival), followed by the protocol using NaOCl (74% cell survival). In contrast, the addition of chlorhexidine led to absence of cell viability. EDTA has similar beneficial effects on dental pulp cells when used as a dentin conditioning agent. When testing the impact of antimicrobial medications on dental pulp cells, researchers showed that calcium hydroxide and lower concentrations of antibiotic pastes are conducive to cell survival and proliferation, but more concentrated forms of antibiotic pastes have detrimental effects.
Microbial Irritants
Although mechanical and chemical irritations are predominantly transient in nature, the most significant cause of inflammation is microbial. Microorganisms present in dental caries are the main source of irritation of the dental pulp and periradicular tissues. Carious dentin and enamel contain numerous species of bacteria, such as Streptococcus mutans, lactobacilli, and Actinomyces spp. More recent studies using molecular techniques have implicated several other genera in the development of carious lesions, including Veillonella, Bifidobacterium, and Propionibacterium spp.
Direct pulp exposure to microorganisms is not a prerequisite for pulpal response and inflammation. Microorganisms in caries produce toxins that penetrate into the pulp through tubules. Studies have shown that even small lesions in enamel are capable of attracting inflammatory cells in the pulp. The initial reaction of the pulp to these irritants is mediated through the innate immune response. This early response to caries results in focal accumulation of chronic inflammatory cells such as macrophages, lymphocytes, and plasma cells. As the decay progresses toward the pulp, the intensity and character of the infiltrate change. When actual exposure occurs, the pulp tissue is infiltrated locally by polymorphonuclear (PMN) leukocytes to form an area of liquefaction necrosis at the site of exposure ( Fig. 4.5 ). After pulp exposure, bacteria colonize and persist at the site of necrosis. Pulpal tissue may remain inflamed for long periods and may undergo eventual or rapid necrosis. This depends on several factors: (1) the virulence of the bacteria, (2) the ability to release inflammatory fluids to avoid a marked increase in intrapulpal pressure, (3) host resistance, (4) the amount of circulation and, an important factor, (5) lymphatic drainage. Yamasaki and associates created pulp exposures in rats and showed that necrosis extended gradually from the upper portion of the pulp to the apical portions. A periapical lesion ensued after pulpal inflammation and necrosis.
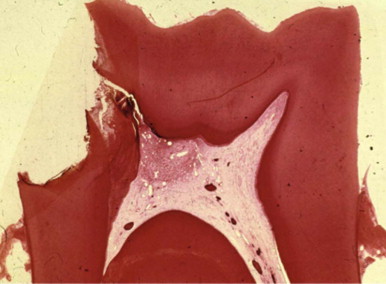
As a consequence of exposure to the oral cavity and to caries, pulp harbors bacteria and their byproducts. The dental pulp usually cannot eliminate these damaging irritants. At best, defenses temporarily impede the spread of infection and tissue destruction. If the irritants persist, the ensuing damage becomes extensive and spreads throughout the pulp. Subsequently, bacteria, or their byproducts, and other irritants from the necrotic pulp diffuse from the canal periapically, resulting in the development of inflammatory lesions ( Fig. 4.6 ).
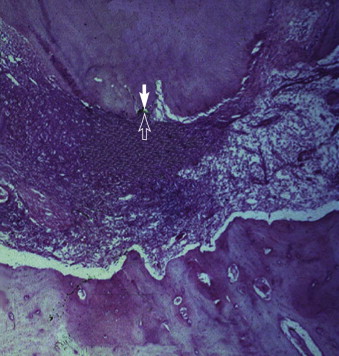
Bacteria play an important role in the pathogenesis of pulpal and periradicular pathoses. A number of investigations have established that pulpal or periradicular pathosis does not develop without the presence of bacterial contamination. Kakehashi and associates created pulp exposures in conventional and germ-free rats. This procedure in the germ-free rats caused only minimal inflammation throughout the 72-day investigation period. Pulpal tissue in these animals was not devitalized, but rather showed calcific bridge formation by day 14, with normal tissue apical to the dentin bridge ( Fig. 4.7, A ). In contrast, infection, pulpal necrosis, and abscess formation occurred by the eighth day in conventional rats ( Fig. 4.7, B ). Other investigators have examined the importance of bacteria in the development of periradicular lesions by sealing noninfected and infected pulps in the root canals of monkeys. After 6 to 7 months, clinical, radiographic, and histologic examinations of teeth sealed with noninfected pulps showed an absence of pathosis in apical tissues, whereas teeth sealed with necrotic pulps containing certain bacteria showed periapical inflammation. The bacteriologic investigation by Sundqvist examining the flora of human necrotic pulps supports the findings of Kakehashi and associates and Möller and coworkers. These studies examined previously traumatized teeth with necrotic pulps, with and without apical pathosis. Teeth without apical lesions were aseptic, whereas those with periapical pathosis had positive bacterial cultures.
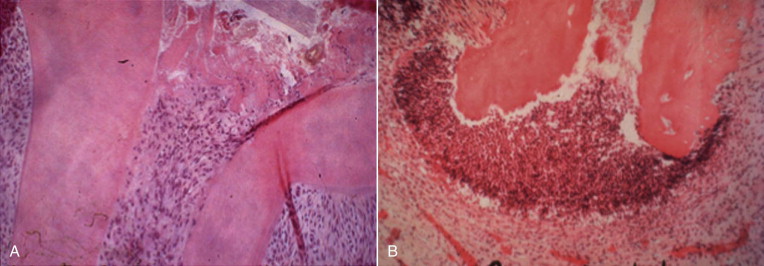
Recent studies have shown a positive correlation between the presence of certain viruses and symptomatic apical pathoses. Periapical lesions that contain cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are more likely to be symptomatic than lesions that do not yield these viruses. Although a direct etiologic role has been suggested by some investigators, this cause and effect relationship has yet to be demonstrated in experimental models.
Several mechanisms have been proposed for identification of bacteria as irritants by the immune system. Detection of these pathogens can occur via interaction between pathogen-associated molecular patterns (PAMPs) and specific receptors broadly identified as pattern recognition receptors (PRRs). PRRs recognize PAMPs and initiate host defenses. G-protein coupled receptors and Toll-like receptors (TLRs) are part of the innate immune response and activate phagocytic functions to allow microbial ingestion. G-protein coupled receptors bind to chemokines, lipid mediators (e.g., platelet-activating factor, prostaglandin E2, and leukotriene B 4 ) or bacterial proteins, causing extravasation of leukocytes and production of bactericidal substances. TLRs are transmembrane proteins that are expressed by cells of the innate immune system. These receptors recognize invading microbes and activate signaling pathways that launch immune and inflammatory responses to destroy the invaders. At least 13 TLRs have been discovered to date with different recognition abilities. Table 4.1 presents some of the currently identified TLRs and their specific interactions.
| PAMP | PRR | Pathogen |
|---|---|---|
| LPS, Lipid A | TLR4 | Gram-negative bacteria |
| Flagellin | TLR5 | Bacteria, flagellum |
| dsRNA | TLR3 | Virus |
| ssRNA | TLR7,8 | Virus |
| CpG DNA | TLR9 | Bacteria, DNA |
| PAMP | PRR | Pathogen |
Pulpal Pathosis
Apart from anatomic configuration and diversity of inflicted irritants, pulp reacts to these irritants as do other connective tissues. A healthy pulp has tremendous capacity to repair itself and to heal; however, often, over time and with exposure to irritants, pulp tissue becomes compromised due to a state of inflammation or fibrosis. Pulpal injury results in cell death and inflammation. The degree of inflammation is proportional to the intensity and severity of tissue damage. Slight injuries, such as incipient caries or shallow cavity preparations, cause little or no inflammation in the pulp. In contrast, deep caries, extensive operative procedures, or persistent irritants usually produce more severe inflammatory changes. Depending on the severity and duration of the insult and the host’s capacity to respond, the pulpal response ranges from transient inflammation (reversible pulpitis) to irreversible pulpitis and then to total necrosis. These changes often occur without pain and without the knowledge of the patient or dentist.
Inflammatory Process
Dentin acts as a physical barrier against irrigants and prevents direct contact between inflammatory cells present in the pulp and the irritants, such as bacteria. Once the thickness of remaining dentin is less than 1.1 to 1.5 mm (e.g., due to caries), the number of inflammatory cells in the pulp increase.
Irritation of the dental pulp results in the activation of a variety of biologic systems, such as nonspecific inflammatory reactions mediated by histamine, bradykinin, and arachidonic acid metabolites. Also released are PMN lysosomal granule products (elastase, cathepsin G, and lactoferrin) ; protease inhibitors (e.g., antitrypsin) ; and neuropeptides (e.g., calcitonin gene–related peptide [CGRP] and substance P [SP]). Phagocytes are not required until direct contact between caries and pulp occurs ; in fact, as long as the dentin thickness remains greater than 2 mm, expression of genes related to PMNs is not up-regulated.
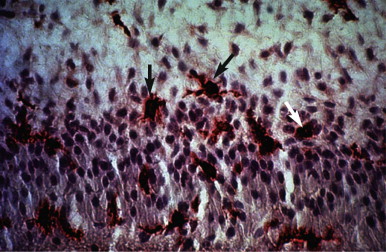
Unlike connective tissue in other parts of the body, normal and healthy dental pulps lack mast cells. However, these cells are found in inflamed pulps ( Fig. 4.8 ). Mast cells contain histamine, leukotrienes, and platelet-activating factors. Physical injury to mast cells, or the bridging of two immunoglobulin E (IgE) molecules by an antigen on their cell surfaces, results in the release of histamine and/or other bioactive substances present in mast cell granules. The presence of histamine in the blood vessel walls and a marked increase in histamine levels indicate the importance of histamine in pulpal inflammation.
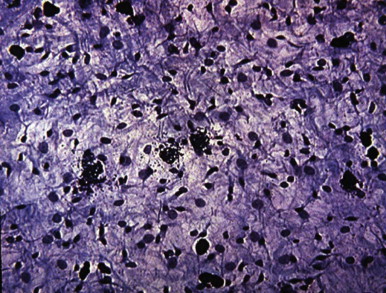
Kinins, which cause many signs and symptoms of acute inflammation, are produced when plasma or tissue kallikreins contact kininogens. Bradykinin, SP, and neurokinin A have been identified in dental pulp tissue using high-performance liquid chromatography. In an in vitro study, bradykinin evoked immunoreactive CGRP (iCGRP) release from bovine dental pulps ; this activity is enhanced by prostaglandin E2 (PGE 2 ). As a result of cellular damage, phospholipase A2 causes release of arachidonic acid from cell membranes. Metabolism of arachidonic acid results in the formation of prostaglandins, thromboxanes, and leukotrienes. Various arachidonic acid metabolites have been found in experimentally induced pulpitis. The presence of these metabolites in inflamed pulps indicates that arachidonic acid metabolites participate in inflammatory reactions of the dental pulp.
The dental pulp is densely innervated with sensory fibers containing immunomodulatory neuropeptides such as SP and CGRP. Studies have shown that denervation of the rat molar pulp, caused by axotomy of the inferior alveolar nerve, results in increased pulp tissue damage and a diminished infiltration of immunocompetent cells. These findings indicate that pulpal nerves are protective in nature and that they may be involved in the recruitment of inflammatory and immunocompetent cells to the injured pulp.
Mild to moderate pulpal injuries result in the sprouting of sensory nerves with an increase in iCGRP. However, severe injuries have the opposite effect, resulting in either reduction or elimination of iCGRP and SP. Experiments indicate that pulpal neuropeptides undergo dynamic changes after injury. In addition, recent studies have shown that stimulation of the dental pulp by caries results in the formation of various interleukins and recruitment of inflammatory cells to the site of injury.
Immunologic Responses
Upon initial invasion of the pulp-dentin complex with microbial irritants, the innate immune system is activated. The innate immune system is immediately available and does not require a complicated method of selecting cells that react to the invaders and not the host, and there is no existence or development of memory. If the innate immune response is unable to eradicate the insult, the adaptive immune system is called into play with cellular and specific antibody responses.
Dendritic cells (DCs) are the master regulators of the immune system and are present in and scattered through normal pulp. In a healthy pulp, the odontoblasts are the cells in direct contact with the overlying dentin. When the pulp is irritated, the odontoblastic processes sense the invasion and elicit recruitment of DCs. DCs phagocytose the invaders and present antigens to stimulate T lymphocytes.
In addition to nonspecific inflammatory reactions, immune responses also may initiate and perpetuate deleterious pulpal changes. Potential antigens include bacteria and their byproducts within dental caries, which directly (or via the dentinal tubules) can initiate various types of reactions. Normal and uninflamed dental pulps contain immunocompetent cells, such as T and B (fewer) lymphocytes, macrophages, and a substantial number of class II molecule-expressing dendritic cells, which are morphologically similar to macrophages. Elevated levels of immunoglobulins in inflamed pulps ( Fig. 4.9 ) show that these factors participate in the defense mechanisms involved in protection of this tissue. Arthus-type reactions do occur in the dental pulp. In addition, the presence of immunocompetent cells, such as T lymphocytes, macrophages, and class II molecule-expressing cells appearing as dendritic cells ( Fig. 4.10 ), in inflamed pulps indicates that delayed hypersensitivity reactions can also occur in this tissue. Despite their protective mechanisms, immune reactions in the pulp can result in the formation of small necrotic foci and eventual total pulpal necrosis.

Lesion Progression
Mild injuries may not result in significant pulpal changes. However, moderate to severe injuries to the pulp result in localized inflammation and the release of a high concentration of inflammatory mediators. An increase in protease inhibitors in moderately to severely inflamed pulps indicates the presence of natural modifiers. As a consequence of the release of a large quantity of inflammatory mediators, increased vascular permeability, vascular stasis, and migration of leukocytes to the site of injury occur. Current research data show that the sensory neuropeptide, CGRP, is responsible for the increase in blood flow during pulpal inflammation.
Elevated capillary pressure and increased capillary permeability move fluids from blood vessels into the surrounding tissues. If removal of fluids by venules and lymphatics does not coincide with the filtration of capillaries, an exudate forms. Pulp is encased in rigid surrounding tissues, forming a low-compliance system; therefore, a small increase in tissue pressure causes passive compression and even complete collapse of the venules at the site of pulpal injury. Pressure increases occur in small “compartmentalized” regions and progress slowly. Therefore, the dental pulp does not degenerate by extensive increases in pressure, with subsequent strangulation.
Pain is often caused by several factors. The release of mediators of inflammation causes pain directly by lowering the sensory nerve threshold. These substances also cause pain indirectly by increasing both vasodilation in arterioles and vascular permeability in venules, resulting in edema and elevation of tissue pressure. This pressure acts directly on sensory nerve receptors.
Increased tissue pressure, the inability of the pulp to expand, and the lack of collateral circulation may result in pulpal necrosis and the development of subsequent periradicular pathosis.
Classification of Pulpal Diseases
Because there is little or no correlation between the histologic findings of pulpal pathosis and clinical symptoms, the diagnosis and classification of pulpal diseases are based on clinical signs and symptoms rather than histopathologic findings. Pulpal conditions can be classified as normal pulp, reversible and irreversible pulpitis, hyperplastic pulpitis, necrosis, and previously treated pulp. Hard tissue responses include calcification and resorption.
Normal Pulp
A tooth with a normal pulp is clinically symptom free and responds normally to vitality tests. Such a tooth does not reveal any radiographic signs of pathosis.
Reversible Pulpitis
By definition, reversible pulpitis is a clinical condition associated with subjective and objective findings indicating the presence of mild inflammation in the pulp tissue. If the cause is eliminated, the inflammation reverses and the pulp returns to its normal state.
Mild or short-acting stimuli can cause reversible pulpitis, such as incipient caries, cervical erosion, or occlusal attrition; most operative procedures; deep periodontal curettage; and enamel fractures resulting in exposure of dentinal tubules.
Symptoms
Reversible pulpitis is usually asymptomatic. However, when present, symptoms usually follow a particular pattern. Application of stimuli, such as cold or hot liquids or air, may produce sharp, transient pain. Removal of these stimuli, which do not normally produce pain or discomfort, results in immediate relief. Cold and hot stimuli produce different pain responses in normal pulp. When heat is applied to teeth with uninflamed pulp, the initial response is delayed; the intensity of pain increases as the temperature rises. In contrast, pain in response to cold in normal pulp is immediate; the intensity tends to decrease if the cold stimulus is maintained. Based on these observations, pulpal responses in both health and disease apparently result largely from changes in intrapulpal pressures.
Treatment
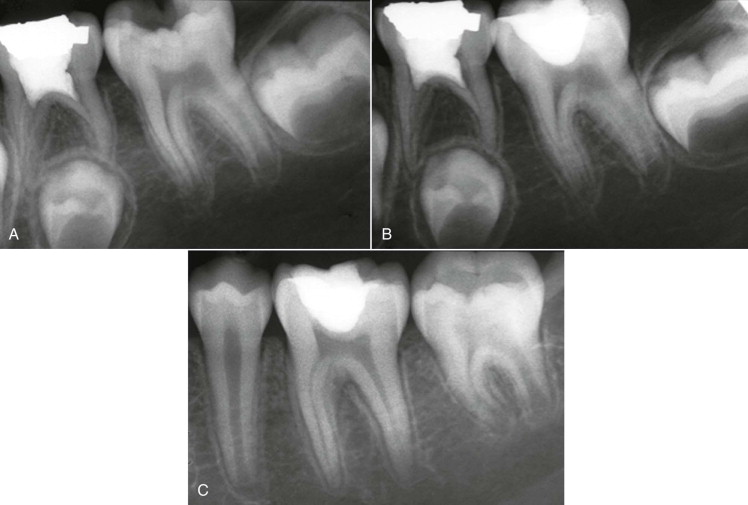
The removal of irritants and sealing and insulating the exposed dentin or vital pulp usually result in diminished symptoms and reversal of the inflammatory process in the pulp tissue ( Fig. 4.11 ). Nevertheless, if irritation of the pulp continues or increases in intensity for the reasons stated earlier, moderate to severe inflammation develops, with resultant irreversible pulpitis and eventually pulpal necrosis.
Irreversible Pulpitis
Irreversible pulpitis may be classified as symptomatic or asymptomatic. It is a clinical condition associated with subjective and objective findings indicating the presence of severe inflammation in the pulp tissue. Irreversible pulpitis is often a sequel to and a progression from reversible pulpitis. Severe pulpal damage from extensive dentin removal during operative procedures or impairment of pulpal blood flow as a result of trauma or orthodontic movement of teeth may also cause irreversible pulpitis. Irreversible pulpitis is a severe inflammatory process that does not resolve, even if the cause is removed. The pulp is incapable of healing and slowly or rapidly becomes necrotic. Irreversible pulpitis can be symptomatic with spontaneous and lingering pain. It can also be asymptomatic, with no clinical signs and symptoms.
Symptoms
Irreversible pulpitis is usually asymptomatic. However, patients may report mild symptoms. Irreversible pulpitis may also be associated with intermittent or continuous episodes of spontaneous pain (with no external stimuli). Pain resulting from an irreversibly inflamed pulp may be sharp, dull, localized, or diffuse and can last anywhere from a few minutes up to a few hours. Localization of pulpal pain is more difficult than localization of periradicular pain and becomes more difficult as the pain intensifies. Application of external stimuli, such as cold or heat, may result in prolonged pain.
Accordingly, in the presence of severe pain, pulpal responses differ from those of uninflamed teeth or teeth with reversible pulpitis. For example, application of heat to teeth with irreversible pulpitis may produce an immediate response; also, occasionally with the application of cold, the response does not disappear and is prolonged. Application of cold in patients with painful irreversible pulpitis may cause vasoconstriction, a drop in pulpal pressure, and subsequent pain relief. Although it has been claimed that teeth with irreversible pulpitis have lower thresholds to electrical stimulation, Mumford found similar pain perception thresholds in inflamed and uninflamed pulps.
Tests and Treatment
If inflammation is confined to the pulp and has not extended periapically, teeth respond within normal limits to palpation and percussion. The extension of inflammation to the PDL causes percussion sensitivity and allows better localization of pain. Root canal treatment or extraction is indicated for teeth with signs and symptoms of irreversible pulpitis.
Hyperplastic Pulpitis
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


