Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Describe pulp protection and pulp therapy.
- 2.
Understand the special physiologic and structural characteristics of the pulp-dentin complex and how they affect the pulpal response to injury.
- 3.
Describe the reparative mechanisms of the pulp, including immune responses and tertiary dentin formation.
- 4.
Describe the effects of dental procedures and materials on the pulp.
- 5.
Appreciate the significance of microleakage and smear layer on pulp response.
- 6.
Describe the indications and procedures for vital pulp therapy.
- 7.
Discuss the effects of pulpal injury in teeth with developing roots.
- 8.
Describe diagnosis and case assessment of immature teeth with pulp injury.
- 9.
Describe the techniques for vital pulp therapy (apexogenesis) and root-end closure (apexification).
- 10.
Describe the prognosis for vital pulp therapy and root-end closure.
- 11.
Describe the technique for pulp revascularization and the goals of regenerative endodontic therapy.
- 12.
Recognize the potential of tissue engineering techniques in regenerating pulpal tissue.
- 13.
Consider restoration of the treated immature tooth.
Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Describe pulp protection and pulp therapy.
- 2.
Understand the special physiologic and structural characteristics of the pulp-dentin complex and how they affect the pulpal response to injury.
- 3.
Describe the reparative mechanisms of the pulp, including immune responses and tertiary dentin formation.
- 4.
Describe the effects of dental procedures and materials on the pulp.
- 5.
Appreciate the significance of microleakage and smear layer on pulp response.
- 6.
Describe the indications and procedures for vital pulp therapy.
- 7.
Discuss the effects of pulpal injury in teeth with developing roots.
- 8.
Describe diagnosis and case assessment of immature teeth with pulp injury.
- 9.
Describe the techniques for vital pulp therapy (apexogenesis) and root-end closure (apexification).
- 10.
Describe the prognosis for vital pulp therapy and root-end closure.
- 11.
Describe the technique for pulp revascularization and the goals of regenerative endodontic therapy.
- 12.
Recognize the potential of tissue engineering techniques in regenerating pulpal tissue.
- 13.
Consider restoration of the treated immature tooth.
Definitions
Pulp Protection
Dental caries represents one of the principal challenges to the health of the dental pulp, although its treatment may well exacerbate the challenge. Cavity preparation and associated procedures, the toxicity of restorative materials and, significantly, the continuing challenge from leakage of bacteria and their products at the margins of restorations can contribute further damage to that caused by the original caries. This may tip the balance from a reversible to an irreversible pulpitis; it also highlights the importance of a holistic approach to management of dental caries, which aims to restore the functional integrity of the tooth while preserving its vitality and protect the pulp from further damage.
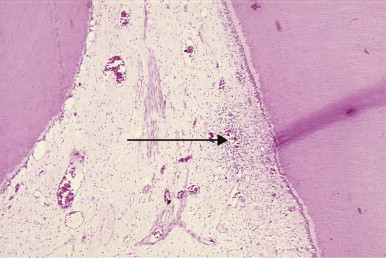
A fundamental consideration in pulp protection is the recognition that infection is a key driver of inflammation, which often determines the outcomes for pulp survival. Thus, the pulp always is likely to be inflamed when bacteria from dental caries are present, and their control should be a significant feature of any treatment planning. Even in teeth with white spot lesions and for which restorative procedures are not indicated, pulpal inflammation is frequently present ( Fig. 2.1 ). When treatment plans are designed for patients who have several teeth with carious lesions, especially when lesions are extensive, a “triage” approach is preferable. In this approach, active caries is removed and good temporary restorations are placed at an early stage, allowing the pulp the maximum opportunity for recovery.
Vital Pulp Therapy
Maintenance of pulp vitality should always be the goal in treatment planning, and considerable interest is developing in the concept of regenerative endodontics for complete or partial pulp tissue regeneration. With mechanical exposure of the dental pulp by trauma or during cavity preparation, the pulp may be protected and its vitality maintained by immediately covering it (pulp capping) and placing a restoration, thereby avoiding root canal treatment. If the exposure is large or seriously contaminated, it may be possible to remove the more superficial diseased part of the pulp (pulpotomy), cap the remaining pulp, and place a restoration. This approach probably has the best prognosis in incompletely formed teeth (especially primary teeth) when the root has not yet reached its full length (apexogenesis); however, it can also be considered for fully formed and permanent teeth with reversible pulpitis. In teeth with necrotic pulps and incomplete root formation, apical closure (but not root elongation) can be obtained by apexification. It is important to recognize, however, that pulp inflammation is a progressive disease, and the use of regenerative approaches to maintain pulp vitality requires a good understanding of the interplay of biologic factors influencing regenerative events, in addition to appropriate case selection. Such approaches may not be suitable for all cases, especially those showing deep pulp inflammation involving the radicular tissue, and the correlation of clinical symptoms with the pathophysiologic status of the dental pulp remains a significant challenge. In the future, tissue engineering may allow replacement of part or all of the pulp with new tissue in the clinical setting.
Regeneration, Revascularization, and Revitalization
Pulp necrosis is clearly a terminal, irreversible pathosis of this tissue. In cases of pulp necrosis after complete tooth maturation, the tooth may survive in a state of normal health after root canal therapy. However, for the immature tooth, this treatment may be difficult to perform, and the tooth may be too weak to withstand normal function because of the thin dentinal wall and short root length. Therefore, techniques to control intracanal infection and induce regrowth of connective tissue that would then promote normal root maturation have been introduced in the past few years. The use of the terms revascularization, revitalization, and regeneration to describe the result of these procedures has been open to some debate in the field. Most authors would use the term revascularization or revitalization, because animal studies have not shown that a functional dental pulp actually regenerates after these techniques.
Table 2.1 presents the principal terms currently used in pulpal protection and vital pulp therapy.
| Term | Definition |
|---|---|
| Pulp cap | Treatment of an exposed vital pulp in which the pulpal wound is sealed with a dental material, such as calcium hydroxide or mineral trioxide aggregate (MTA), to facilitate the formation of reparative dentin and maintenance of a vital pulp. |
| Direct pulp cap | A dental material placed directly on a mechanical or traumatic vital pulp exposure. |
| Step-wise caries excavation | Incremental removal of caries over a period of time to allow pulpal healing and to minimize exposure |
| Pulpectomy (pulp extirpation) | The complete surgical removal of the vital pulp. |
| Pulpotomy (pulp amputation) | Surgical removal of the coronal portion of a vital pulp as a means of preserving vitality of the remaining radicular portion; pulpotomy usually is performed as an emergency procedure for temporary relief of symptoms or as a therapeutic measure. |
| Partial pulpotomy (shallow pulpotomy; Cvek pulpotomy) | Surgical removal of a small diseased portion of vital pulp as a means of preserving the remaining coronal and radicular pulp tissues. |
| Apexification | Induction of a calcified or an artificial barrier in a root with an open apex or the continued apical development of an incompletely formed root in teeth with a necrotic pulp. |
| Apexogenesis | A vital pulp therapy procedure performed to enable continued physiologic development and formation of the root end; the term frequently used to describe vital pulp therapy that encourages the continuation of this process. |
| Pulp regeneration | The ability to recreate lost or damaged pulp tissue |
| Pulp revascularization | The restoration of blood supply in the pulp space |
| Pulp revitalization | Recreation of vital connective tissue in the pulp space |
Iatrogenic Effects on the Dental Pulp
Local Anesthesia
When most local anesthetics containing vasoconstrictors are used in restorative dentistry, the blood flow to the pulp is reduced to less than half of its normal rate. In the case of lidocaine with epinephrine, this effect is entirely a result of the vasoconstrictor. In procedures on teeth with pulps that are already compromised, this may be an additional stressor. A healthy pulp may survive episodes of ischemia lasting for 1 hour or longer. An already ischemic pulp subjected to severe injury may hemorrhage (blush) when subjected to trauma such as that associated with full crown preparation without the use of an adequate coolant.
Cavity/Crown Preparation
An appreciation of the cellular structure of the dentin-pulp complex is critical for cavity/crown preparation if further tissue injury is to be minimized and pulp vitality maintained. This is elegantly illustrated in Fig. 2.2 , which demonstrates the intimate contact between odontoblasts, through their processes, and the dentin matrix, highlighting the communication between these cells and their environment.
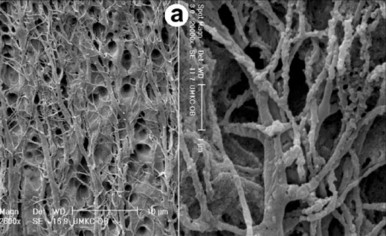
Any surgical intervention to the dentin during cavity preparation may result in some degree of injury to the odontoblasts and their processes. Although this cannot be avoided during cavity preparation, it is important to recognize the consequences of such treatment and to minimize the extent of injury. It is also important to recognize that dentin matrix is not comprised simply of structural components (e.g., collagen and mineral); rather, it also contains a diverse mixture of biologically active molecules, including growth factors, cytokines, and other constituents. Both matrix dissolution during the carious process and the cutting and etching of the dentin during cavity preparation can lead to release of these molecules, with the consequent potential for stimulation of cellular responses in the pulp.
Heat
Frictional heat is produced whenever a revolving bur or stone contacts tooth structure. Until the advent of the high-speed handpiece, enamel and dentin preparation involved heavy torque, low rotational speeds, and steel burs that were not cooled with water. Consequently, vital dentin was often scorched, and pulps were injured as a result of extreme heat ( Fig. 2.3 ).
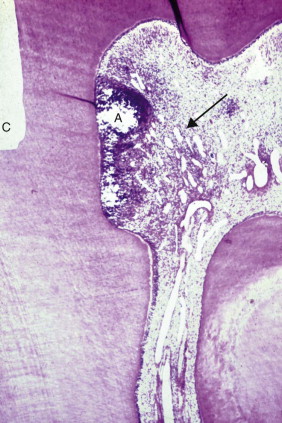
Dentin is an effective insulator; for this reason, careful cutting with adequate cooling is less likely to damage the pulp unless the thickness of the dentin between preparation and pulp is less than 1 mm. Even then, the inflammatory response may be mild ( Fig. 2.4 ). The greatest amount of frictional heat is generated with a large diamond stone when teeth are prepared for a full crown, and the pulp is particularly at risk of injury. The heat generated may also have a desiccating effect by “boiling” away dentinal tubule fluid at the dentin surface.

The “blushing” of dentin during cavity or crown preparation is thought to be due to frictional heat resulting in vascular injury (hemorrhage) in the pulp. The dentin takes on an underlying pinkish hue soon after the operative procedure, reflecting significant vascular injury. Crown preparation performed without the use of a coolant leads to a marked reduction in pulpal blood flow, presumably because of vascular stasis and thrombosis. The amount of heat produced during cutting is determined by the sharpness of the bur, the amount of pressure exerted on the bur or stone, and the length of time the cutting instrument contacts the tooth structure. The safest way to prepare tooth structure is to make sure the bur-dentin interface is constantly wet.
The use of laser beams to fuse enamel and reduce the likelihood of carious invasion has been suggested. Different lasers with different energy levels may also be used to remove caries. Lasers generate heat and increase the intrapulpal temperature. The heat generated varies with a number of parameters, but can be reduced by water cooling to a level similar to that for a water-cooled high-speed drill.
Cavity Depth/Remaining Dentin Thickness
Dentin permeability increases exponentially with increasing cavity depth, because both the diameter and density of dentinal tubules increase with cavity depth ( Fig. 2.5 ). Thus the deeper the cavity, the greater the tubular surface area into which potentially toxic substances can penetrate and diffuse to the pulp. The length of the dentinal tubules beneath the cavity is also important. The farther substances diffuse, the more they are diluted and buffered by the dentinal fluid.
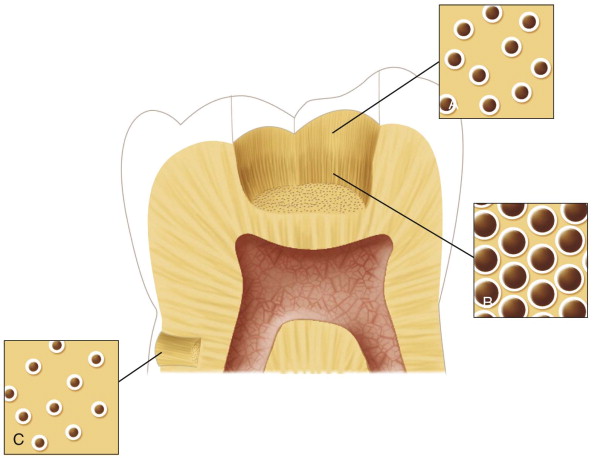
A remaining dentin thickness of 1 mm is often regarded as sufficient to shield the pulp from most forms of irritation. In noncarious teeth, tertiary reactionary dentin is formed most rapidly when the remaining dentin thickness is between 0.5 and 0.25 mm. With a narrower remaining dentin thickness, odontoblast survival is compromised, and any regenerative response would likely involve reparative dentin formation by newly differentiated cells.
The effects of remaining dentin thickness on cellular responses, however, are not only a function of the diffusion of noxious stimuli to the pulp. The morphology of the odontoblast processes is of a more tapered shape, with greater thickness near the cell body of the odontoblast. Deeper cavity preparations sever the odontoblast processes in their regions of greater thickness; this affects the cell’s attempts to restore its membrane integrity and increases the risk of a cell leaking its contents. Cells have well-developed mechanisms for responding to mechanical stress and repairing minor breaks in the integrity of their plasma membranes; however, failure of these mechanisms can lead to disease and loss of cell viability.
Cavity Drying and Cleansing
A prolonged blast of compressed air aimed onto freshly exposed vital dentin causes a rapid outward movement of fluid in patent dentinal tubules through strong capillary forces. Rapid outward flow of fluid in the dentinal tubules stimulates nociceptors in the dentin pulp, thus producing pain. Rapid outward fluid movement may also result in odontoblast displacement ( Fig. 2.6 ). Odontoblasts are dislodged from the odontoblast layer and drawn outward into the tubules, where they undergo autolysis and disappear. Providing the pulp has not been severely injured, displaced odontoblasts may be replaced by new cells derived from stem/progenitor cells deeper in the pulp. In this way, the odontoblast layer is reconstituted by “replacement” odontoblast-like cells capable of producing tertiary reparative dentin. Although drying agents containing lipid solvents, such as acetone and ether, have been used to clean cavity floors, their rapid rate of evaporation can produce strong hydrodynamic forces in the tubules, causing odontoblast displacement. Cavities should be dried with cotton pellets, and only short blasts of air should be carefully applied, rather than harsh chemicals. Toxic antibacterial agents are not advisable for use on the cavity floor due to the risk of injury to the pulp or their limited broad-spectrum specificity to the microflora present. A new generation of antibacterial bonding agents for use with resin-bonded composites is emerging, and such approaches may have merit in the control of residual bacteria. In contrast, use of antibacterial agents is of paramount importance to successful outcomes for root canal therapy, although even in the absence of vital pulp tissue within the root canal, care is required to avoid leakage of potent antibacterial agents into the periapical tissues, where they may compromise tissue vitality.
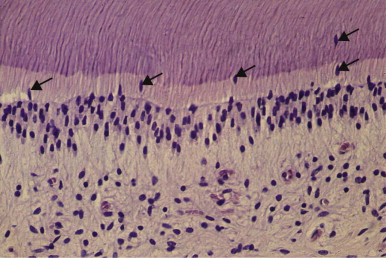
Etching Dentin/Smear Layer Removal
Cutting dentin produces a smear layer on the cut surface consisting of fragments of microscopic mineral crystals and organic matrix debris, which may interfere with the adherence of adhesive restorative materials. Acidic cavity-cleansing products and chelating agents are often used to remove the smear layer, but their use is mainly justified only with the placement of an adhesive restoration. The smear layer does have one desirable property; by blocking the orifices of dentinal tubules, the smear plugs greatly decrease the permeability of dentin. Although the smear layer is largely impervious to bacteria, it is not a barrier to bacterial products. Complete dissolution of the smear layer opens the dentinal tubules, significantly increasing the permeability of dentin. If the dentin is left unsealed, the diffusion of irritants to the pulp may intensify and prolong the severity of pulpal reactions.
Traditionally, cavity etchants have been used based on their physicochemical properties of smear layer removal and cavity cleansing. It is now apparent, however, that they may also locally release some of the biologically active molecules contained within the dentin matrix. Release of these molecules can signal the cellular responses, leading to stimulation of regenerative events ; therefore, it is important to consider the use of cavity etchants within this broader context. This has implications for the application time of these etchants, which reflects a compromise between achieving optimal smear layer removal and maximizing stimulation of regenerative cellular responses.
Other Restorative Procedures
Pulp damage may result from pinhole preparation or pin placement. Coolants do not reach the depth of the pin preparation. During pinhole preparation, there is always the risk of pulp exposure. Furthermore, friction-locked pins often produce microfractures that may extend to the pulp, subjecting the pulp to irritation and the effects of microleakage.
Rubber-base and hydrocolloid materials do not injure the pulp. However, temperatures of up to 52°C have been recorded in the pulp during impression taking with modeling compound, and such temperatures may damage the pulp, especially if it is already inflamed.
Cooling is strongly recommended when provisional crowns are fabricated directly because of the exothermic reaction of autopolymerizing resins. The temporary crown/cement should be left in place for a short period; temporary cements are not stable and eventually wash out. Microleakage around temporary crowns is a common cause of postoperative sensitivity. During the cementation of crowns, inlays, and bridges, strong hydraulic forces may be exerted on the pulp as cement compresses the fluid in the dentinal tubules.
Continuous polishing of amalgam or other metallic restorations with rubber cups at high speeds causes a damaging temperature increase of up to 20°C. Therefore, polishing with rubber wheels, points, or cups should be performed at low speeds using intermittent pressure and a coolant.
The use of burs to remove metallic restorations can produce very high levels of frictional heat. A coolant, such as water spray or a combination of water and air, avoids a burn lesion in the pulp.
Postrestorative Hypersensitivity
Many patients complain of hypersensitivity after a restorative procedure as a result of cumulative effects that irritate the pulp. Discomfort is usually of short duration. If the pain is prolonged, a preexisting pulpitis may have been exacerbated. If it is delayed in onset by days, the cause may be microleakage of bacterial irritants under a poorly sealed restoration. The absence of postoperative sensitivity after restoration with modern composites of both Class I and Class II preparations has been demonstrated in clinical studies, suggesting that variations in technique may be responsible for the anecdotal reports. The use of hydroxymethacrylate/glutaraldehyde “desensitizer” does not reduce the incidence of sensitivity. Self-etching, self-priming dentin bonding systems reduce the incidence of sensitivity after the restoration of deep carious cavities.
One important clinical factor in the placement of resin restorations is the control of moisture. Applying a dental dam during the procedure is thought to help in moisture control. However, there are situations in which the restoration extends subgingivally. Proper moisture control for bonding of the restoration is difficult to achieve in these situations.
If pain is evoked by biting on a recently restored tooth, an intracoronal restoration may be exerting a strong shearing force on the dentin walls of the preparation. It is more likely to be caused by an injury to the periodontal ligament as a result of hyperocclusion. Hyperocclusion from an extracoronal restoration does not injure the pulp but may cause a transient hypersensitivity.
Dental Materials
Microleakage
The most important characteristic of any restorative material in determining its effect on the pulp is its ability to form a seal that prevents the leakage of bacteria and their products onto dentin and then into the pulp.
Cytotoxicity
Certain restorative materials are composed of chemicals that have the potential to irritate the pulp. However, when these materials are placed in a cavity, the intervening dentin usually neutralizes or prevents leachable ingredients from reaching the pulp in a high enough concentration to cause injury. For example, eugenol in zinc oxide–eugenol (ZnOE) is potentially irritating but very little can reach the pulp. Phosphoric acid is a component of silicate and zinc phosphate cements and was thought to be highly injurious to the pulp. However, the buffering capacity of dentin greatly limits the ability of hydrogen ions to reach the pulp. It is now clear that the problems that occurred after use of these materials were a result of their high degree of shrinkage and subsequent microleakage.
Clearly, the thickness and permeability of dentin between a material and the pulp affect the response to the material. In addition, the penetration of some materials through dentin may be limited by the outward flow of fluid through the tubules, which is increased if the pulp is inflamed. This factor has been overlooked in many in vitro studies investigating the passage of materials through dentin.
Many cytotoxicity studies examine isolated cell types in culture and do not take into account the immunocompetent cells present in the intact pulp. Materials may have a differential effect on these cells by either stimulating or inhibiting their activity.
Materials are more toxic when they are placed directly on an exposed pulp. Cytotoxicity tests carried out on materials in vitro or in soft tissues may not predict the effect of these materials on the dental pulp. The toxicity of the individual components of a material may vary. A set material may differ in toxicity from an unset material. The immediate pulpal response to a material is much less significant than the long-term response. A few days after placement, the pulp may show a strong inflammatory response. A few months later, the inflammatory response may subside and repair occurs. A good measure of long-term response is the thickness of tertiary dentin laid down by the affected pulp ( Fig. 2.7 ).
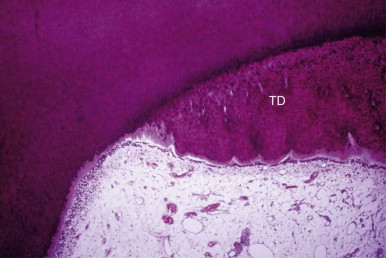
Depth of Preparation
Deep cavity preparations are likely to destroy odontoblasts. These are replaced by new odontoblasts that often form an irregular, reparative dentin that has few tubules (see Figure 1-8, A ). Deep preparations also limit the amount of dentin that separates the cavity from the pulp, thus increasing the deleterious effects of biologic and chemical irritants.
Desiccation by Hygroscopy
Some hygroscopic materials may cause injury by withdrawing fluid from dentin. However, the relationship between the hydrophilic properties of materials and their effect on the pulp is minimal. Moisture absorbed by materials is probably much less than that removed from dentin during cavity drying, which is a procedure that produces a small amount of pulpal inflammation.
Specific Materials
Zinc Oxide–Eugenol
ZnOE has many uses in dentistry. It has a long history as a temporary filling material, cavity liner, cement base, and luting agent for provisional cementation of castings. Before the introduction of calcium hydroxide, ZnOE was the material of choice for direct pulp capping.
Eugenol, biologically the most active ingredient in ZnOE, is a phenol derivative and is toxic when placed in direct contact with tissue. It also has antibacterial properties. Eugenol’s usefulness in pain control is attributed to its ability to block the transmission of nerve impulses. Researchers have found that a thin mix of ZnOE significantly reduces intradental nerve activity when placed in a deep cavity preparation in cats’ teeth; however, a dry mix of ZnOE has no effect.
When included in cements to temporize crown preparation, some eugenol does reach the pulp, but the amounts are small and unrelated to remaining dentin thickness. “Desensitizing” agents do not seem to reduce the penetration. The release of eugenol is by a hydrolytic mechanism, which depends on the presence of water. With little water available, release is low.
The most important property of ZnOE is that it prevents microleakage of bacterial cells, thereby reducing hypersensitivity and providing antimicrobial properties.
Restorative Resins
Early adhesive bonding and resin composite systems contract during polymerization, resulting in gross microleakage and bacterial contamination of the cavity. Bacteria on cavity walls and within axial dentin are associated with moderate pulpal inflammation. Over a period of time, some composites absorb water and expand; this tends to compensate for initial contraction. To limit microleakage and improve retention, enamel margins are beveled and acid etched to facilitate mechanical bonding. Compared with unfilled resins, the newer resin composites present a coefficient of thermal expansion similar to that of tooth structure. With recently developed hydrophilic adhesive bonding composite systems, the problem of marginal leakage appears to have been diminished. A large, practice-based study has recently shown that the mean annual failure rate for resin-composite restorations was 2.9% and for amalgams it was 1.6%.
Glass Ionomer Cements
Glass ionomer cements were originally used as esthetic restorative materials, but these cements are now being used as liners, luting agents, temporary restorative materials, and pulp capping agents (sometimes in conjunction with calcium hydroxide). The incidence of severe pulpal inflammation or necrosis caused by glass ionomer cement on exposed healthy pulps is similar to that for calcium hydroxide but greater than that for composite resins. When placed in cavities in which the pulp is not exposed and the remaining dentin thickness is narrow (0.5 to 0.25 mm), both calcium hydroxide and composite resin show faster deposition of tertiary dentin than glass ionomer cements.
When glass ionomer cements are used as a luting agent, the pulpal response is similar to that of polycarboxylate and zinc orthophosphate (ZnOP) cements. Studies have shown that when glass ionomer cements are used with appropriate technique, the incidence of sensitivity is no greater than with other commonly used luting agents.
Amalgam
Amalgam alloy is still a widely used material for restoring posterior teeth. Shrinkage during setting results in microleakage. This microleakage decreases as corrosion products accumulate between restoration and cavity walls, and it can be reduced by the use of liners. Amalgam is the only restorative material in which the marginal seal improves with time. Esthetics and public concern with the mercury content of amalgams have led to an accelerated use of composite resins as posterior restorative materials. Their use is more technique sensitive than amalgams. In deep cavities in posterior teeth, composites are associated with more pulpal injury than amalgams because of microleakage. At least in part, this may be due to the difficulty with moisture control in some sites, as noted before.
Orthodontic Tooth Movement
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


