Fig. 2.1
(a–d) MicroCT reconstruction of a rodent mandibular molar showing in (a) the outer surface of the first mandibular molar; in (b) a virtual section showing, namely, the enamel, dentin, pulp, and cementum; and in (c, d) the 3D reconstruction
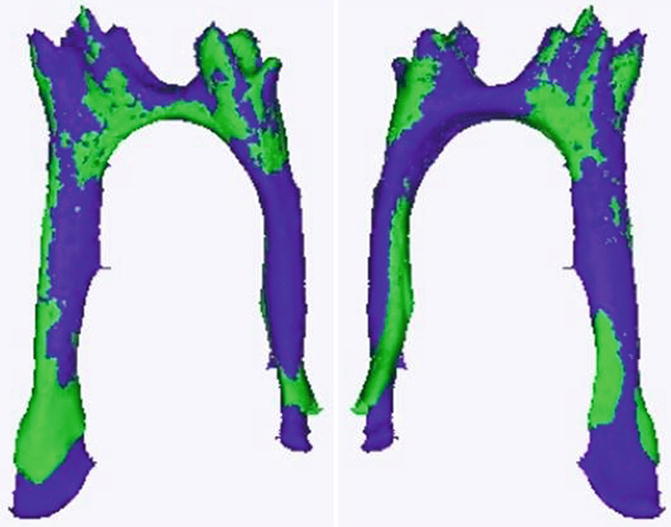
Fig. 2.2
3D reconstitution of the pulp of a wild-type (in green) molar compared to the pulp of a 5HT2BR KO mouse in purple. The pulp of the KO mice is longer and wider. The comparison between the age-matched WT and KO mice shows that dentinogenesis is altered by the receptor deletion
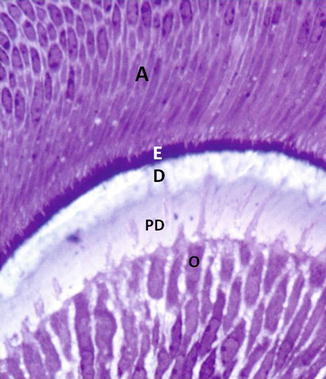
Fig. 2.3
Postmitotic secretory odontoblasts (O) are implicated in the secretion of predentin (PD) and in dentin (D) mineralization. At this early stage of odontogenesis, a thin layer of forming enamel is secreted by the secretory ameloblasts (A)
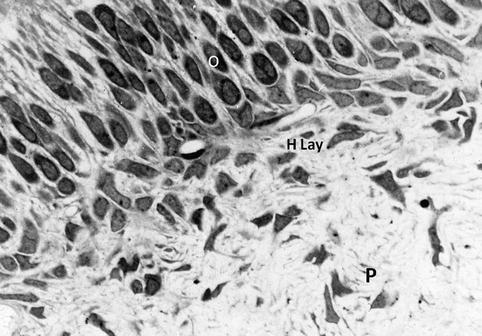
Fig. 2.4
From the outer layer (upper part) to the inner zone (lower zone), odontoblast cell bodies (O) and Hoehl’s cell layer (H Lay) are located at the pulp periphery (P)
2.2 General Organization
In adult teeth, odontoblasts and Hoehl’s cells form a superficial layer at the periphery of the pulp, contributing to the configuration an outer border lining the dental pulp. Odontoblasts are implicated in the production of the extracellular predentin/dentin matrix and, subsequently, they are involved in the dentin mineralization process. Odontoblasts and Hoehl’s cells take origin from the neural crests. The progenitors migrate toward the first branchial arch and contribute to the formation of tooth germs. They differ substantially from the pulp cells, both from a developmental point of view, displaying specific composition and functionality. Additional differences were suggested between the cell-free zone presumably located at the pulp surface, beneath the subodontoblastic cell layer, now recognized as a fixation artifact, and the cell-rich zone, containing progenitor cells that display plasticity and pluripotent capabilities.
This points out the complexity of the odontoblast, Hoehl’s cells, and pulp layers. Although reference is often made in the literature to the existence of a so-called dentino-pulpal complex, much evidence denies this notion. This working hypothesis refers apparently to physiopathologic pain perception, pulpitis, and dental treatments but is not actually based on any biological proof. This statement is clearly questionable both with respect to anatomical and biological specificities, gene and transcription factors expression, and with respect to the embryological origin of the tissues, the neural crest-derived tissues differing from mesenchymal branchial arch [1].
The dental pulp is formed by cells which are implicated in the secretion and reorganization of a collagen-rich extracellular matrix (ECM). Pulp cells play a crucial role in the synthesis and in the ECM molecules remodeling:
(i)
In the dental pulp, a series of characteristic cells have been identified. The pulp stromal fibroblasts, also named pulpoblasts by Baume [2], constitute the most abundant pulp cell population. In addition, other cell lineages have been recognized in the pulp: specifically progenitors (also named stem cells, or side population) and neuronal, vascular, and immune system cells. Resident structural cells are permanently subjected to renewal and apoptosis. This cell population includes stem cells, which are acting as progenitors. According to some researchers, in the dental pulp, a ~9 % positivity was found for STRO-1, a stem cell marker, whereas according to Kenmotsu et al. [3] between 0.11 and 0.40 % of the pulp cells are stem cells (Figs. 2.5 and 2.6).
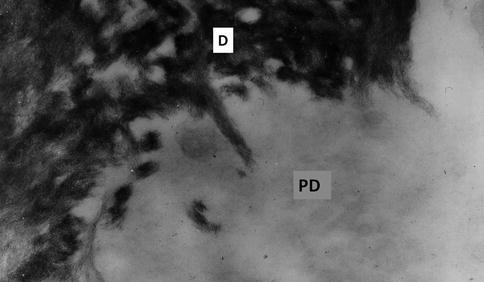
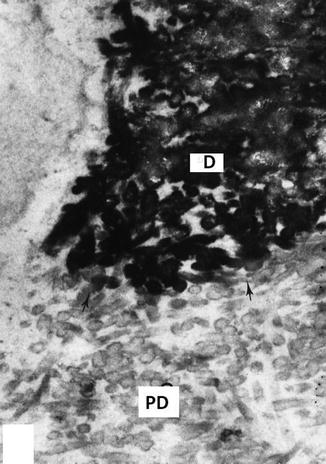

Fig. 2.5
Unstained ultrathin section. Dentin-predentin junction. Hydroxyapatite needlelike crystals are located along collagen fibrils at the mineralizing front in dentin (D), whereas no mineral is detectable in the predentin (PD)

Fig. 2.6
Junction between dentin (D) and predentin (PD) stained with the phosphotungstic acid/chromic acid mixture. Dentin loaded by the dentin sialophosphoprotein is heavily electron dense, whereas in the predentin (PD), thin rings around individual collagen fibrils revealed electron-dense phosphorylated proteins
(ii)
In addition, a few nonresident cells are found. They migrate from the blood or take origin in the bone marrow and/or other non-dental tissues. They migrate and penetrate within the dental pulp through the apical foramen.
Different adjacent domains contribute to the pulp organization, each domain bearing its own specificity. The dental pulp includes a mosaic of territories, varying in the crown and the root, and in the central part versus the peripheral pulp as well.
2.3 Odontoblasts
The postmitotic odontoblasts are implicated in dentinogenesis. A cell body and a cell process form each odontoblast. Cell bodies are grouped in four to five rows parallel to the tooth surface at the periphery of the pulp.
Each cell body comprises a basal third containing an abundant rough endoplasmic reticulum (RER) and mitochondria. A nucleus is also located in this basal third. Many cells display cilia. Cytoskeletal proteins direct the shape and functionality of the cell bodies. In the central part, dictyosomes are located in the supranuclear area. Multivesicular vesicles and lysosomal electron-dense vesicles, with variable content, are also detected. The RER located along the lateral borders contributes to the MEC synthesis. The Golgi apparatus and multivesicular bodies play role in the terminal steps of ECM synthesis and, after re-internalization of molecules cleaved by metalloproteases (MMPs), in the control of degradation. In the distal cell body, the RER is interrupted at halfway. Small mitochondria are grouped near the place where the processes take origin.
The odontoblast main processes display bundles of cytoskeletal proteins such as microtubules, intermediary filaments identified as vimentin and nestin, and actin microfilaments. These later contribute to a sub-plasmalemmal undercoat. Secretory vesicles and acid phosphatase-rich endocytotic vesicles (coated vesicles) are implicated in active secretion and/or reabsorption. Odontoblast processes cross the predentin and penetrate inside dentin tubules either in the inner third or along the whole dentin length, up to the dentinoenamel junction. The diameter of odontoblast lateral branchings is thinner. They establish connections between tubules, penetrating in minute tubules and crossing the whole thickness of the hypermineralized peritubular dentin. The lateral branches do not contain nestin, but only vimentin and actin.
Odontoblasts have a limited lifespan and when they mature and become aged, they start to be loaded by lysosomes and autophagic vacuoles. Then, they become apoptotic cells [4]. The number of odontoblast inside the outer cell layers is gradually reduced. The cells become smaller, and eventually they are reduced to a single layer. Differentiating Hoehl’s cells, which behave as odontoblast second generation, presumably renews them [5].
During the teeth formation, odontoblasts are implicated in the synthesis and secretion of the dentin extracellular matrix (Figs. 2.7 and 2.8a–c). The cells issued from the neural crest migrate toward the first branchial arch and settle near the dental lamina of the mandibular, maxillary, and nasofrontal buds. In front of the epithelial dental lamina, the pre-odontoblasts divide and migrate from the central to the outer part of the pulp. The number of mitosis is fixed depending on the species. During the last division of pre-odontoblast, an asymmetric division occurs. The taller cells establish a limited contact with the basement lamina (BM). These pre-odontoblasts become pre-secretory pre-polarized odontoblasts. The smaller cells are located some distance away from the basement membrane. They are clustered in Hoehl’s cell layer. At an early stage, odontoblasts contribute to the formation of the coronal dentin. They are implicated in the synthesis of an extracellular matrix, implicated in dentin mineralization. The first outer dentin layer formed is atubular. This layer is also named mantle dentin in the crown. Afterward they are involved in the physiologic primary and secondary dentin formation.
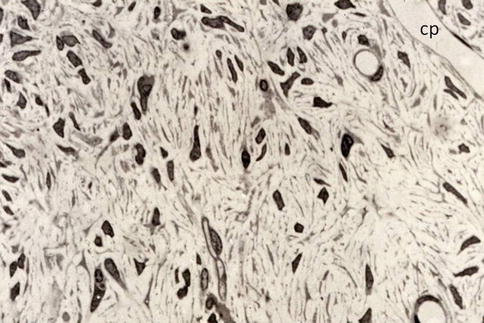
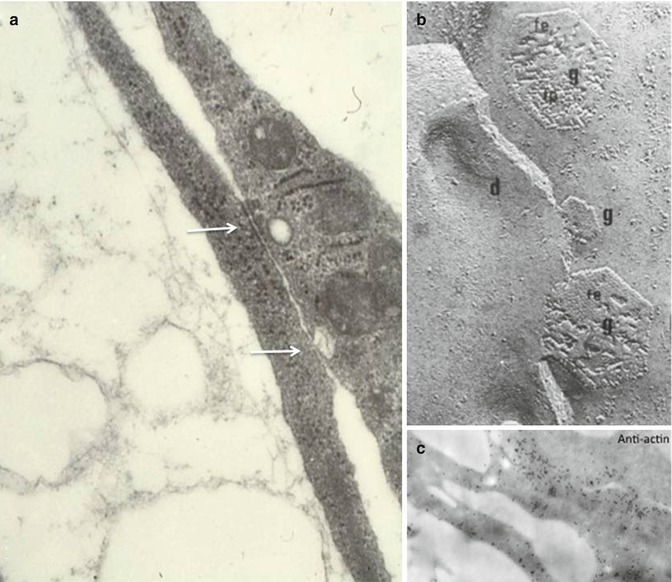

Fig. 2.7
Pulp cells include a heterogeneous population of fibroblasts (pulpoblasts) and endothelial cells of capillaries (cp)

Fig. 2.8
(a) Intercellular junctions of the gap junction and desmosome-like type between pulpoblasts (white arrows). Thin collagen fibrils are detectable in the extracellular spaces. (b) Freeze fracture replica obtained after rapid freezing-freeze substitution. G gap junction (e or f faces), d desmosome-like structure. (c) Plasmalemmal undercoat after immunogold labeling with an anti-actin antibody
After crown completion, during the next stage, the root starts to be formed. Pre-odontoblasts migrate from the central dental pulp toward the periphery, just beneath Hertwig’s epithelial root sheath. Differentiation of pre-odontoblasts precedes the terminal differentiation of these cells into radicular odontoblasts. There is still a debate to clarify if the inner layer of Hertwig’s epithelial layer is susceptible to transdifferentiate. Due to phenotypic changes, epithelial cells become cementoblasts and afterward may become cementocytes. As an alternative possibility, pre-cementoblasts issued from the dental follicle may slide between the unbound cells of Hertwig’s epithelial root sheath. Intercellular junctions are disrupted, and intercellular spaces enlarge. Mesenchymal cells of the dental follicle infiltrate the spaces of the root sheath and come in contact with the outer dentin surface of the root, where they acquire the final cementoblast phenotype. The dental follicle contributes to the formation of the bonny socket and of the dental ligament. Subsequently the root formation starts, preceding tooth eruption and pulp lengthening. In the dental pulp, stem cells or odontoblast progenitors differentiate and contribute to root dentinogenesis. After the outer dentin layer(s) formation (Tomes’ granular layer and/or amorphous Hopewell-Smith layers), the root circumpulpal dentin starts its initial construction either as tubular dentin or appearing as a fibrodentin structure.
After tooth formation, odontoblasts are located at the periphery of the pulp keeping a pseudostratified palisade structure. Primary dentinogenesis occurs during the early secretory period of tooth formation. The synthesis and secretion of ECM are gradually reduced and autophagic activities increase. Thereafter comes a period of decreasing activity for the odontoblasts. The primary dentinogenesis starts just after the formation of the mantle dentin and stops when the teeth become functional, with occlusal pressures. As postmitotic cells, odontoblasts are implicated in the formation and maintaining of dentin. Quiescent odontoblasts are implicated in the formation of secondary dentin during all life span. During aging, odontoblast develops an autophagic-lysosomal system organized in large vacuoles, which are acid phosphatase positive. The lysosomal markers LC3 and LAMP2 are indicative of a dynamic autophagic activity, implicated in the turnover and degradation machinery. Accumulation of lipofuscin was seen within lysosomes [4]. Reactionary (or tertiary) dentin is produced in response to a carious lesion, to abrasion, or a noxious reaction to dental materials.
2.3.1 Subodontoblastic Layer/Hoehl’s Cell Layer
Presumably Hoehl’s cells take origin from the last pre-odontoblast cell division (Figs. 2.5 and 2.6). Odontoblasts form originally a structured layer including about four rows of cells. During tooth maturation and aging, the number of odontoblasts is gradually reduced, due to apoptotic events, and finally they appear as a thin cell monolayer. There is high probability that odontoblasts have a limited life span. In this context, subodontoblastic cells are implicated in cell replacement. Their terminal differentiation is activated, and they become what have been named second-generation odontoblasts. This hypothesis is reinforced by the fact that Hoehl’s cells express high alkaline phosphatase activity. The majority of subodontoblastic cells express Thy-1, a cell surface marker of stem cells and progenitors. The capacity of Thy-1 to be expressed by the subodontoblastic cells was evaluated following stimulation with BMP-2. Thy-1 positive cells showed accelerated induction of ALP activity. They formed alizarin red-positive mineralized nodules and induced the formation of bone-like matrix. Hosoya et al. [6] concluded that the subodontoblastic cells have the ability to differentiate into hard tissue-forming cells, and consequently they may serve as a source of odontoblastic cells. To conclude, odontoblasts and the subodontoblastic layer are both involved in dentinogenesis. These cells contribute to reactionary dentin formation, whereas pulp cells are implicated in reparative dentin formation.
2.4 Stromal Fibroblasts (or Pulpoblasts)
2.4.1 Resident Cells
2.4.1.1 Phenotypic Characterization
Most of the pulp cells are resident cells. They play a structural role, shaping the construction of the pulp and acting as feeder cells. These cells were identified on the basis of their morphology. Pulp fibroblasts (or pulpoblasts) are elongated cells, with narrow diameter and long protracted processes. In the dental pulp, fibroblasts (or pulpoblasts) are fusiform cells, bound by intercellular desmosome-like, gap junctions and a few tight junctions. A small number of stem cells, or pulp progenitors, are included in this group (Figs. 2.9, 2.10, and 2.11).
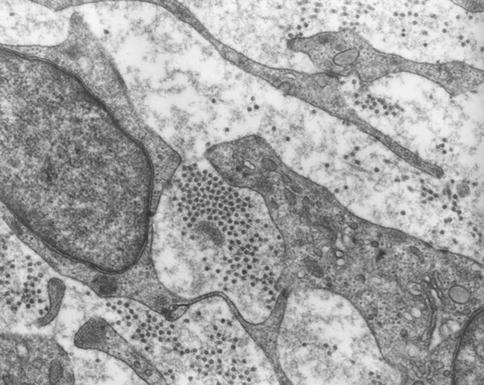
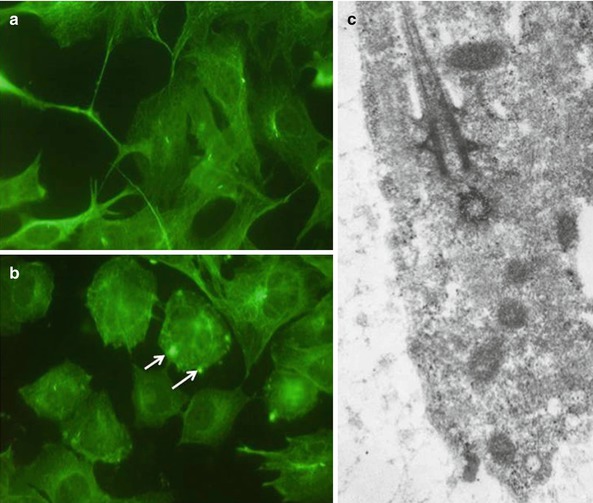
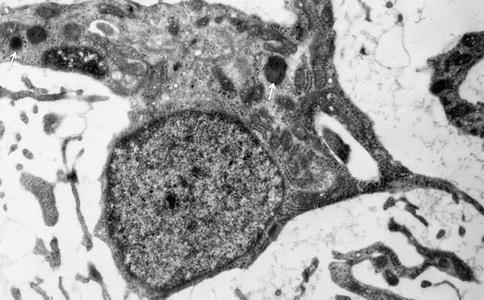

Fig. 2.9
Pulp fibroblasts. In the extracellular spaces, different types of collagen fibrils are found, corresponding to type I (aggregates of round structures) and type III collagens (ramified thin and thick fibrils less electron dense)

Fig. 2.10
(a–c) Immunostaining of cilium using an alpha acetyl tubulin antibody (white arrows) (a, b). In (c), a cilium and basal body in an odontoblast cell body. This structure formed by bundles of microtubules contributes to act as a receptor in the odontoblast cell bodies

Fig. 2.11
Pulp fibroblasts are also implicated in lysosomal (white arrow) and catabolic activities
A few other cells take origin and migrate from blood circulation, bone marrow, and other non-dental tissues. This second group constitutes the nonresident cells, also designed as migrating mesenchymal cells. They penetrate the pulp by the apical part and invade the tissue.
There is much evidence that resident and nonresident cells have a limited life span. In order to keep a continuous volume and maintain the diverse functions of the tissue, a constant renewal of pulp cells is mandatory. An experimental approach was conducted on young rats, using a essential fatty acid-deficient diet (EFAD) from day 0 to day 21 (Group I), whereas another group received after birth a normal diet, followed at day 21 for 4 weeks by a deficient diet (Group II). These two groups were compared with a group of rats receiving a normal diet (Group III) and with a per-fed group (a group of rats receiving a reduced food intake) (Group IV) [8]. The number of cells/mm2 was scored in the central part and in the subodontoblastic lateral areas. In groups I and II, the cell density was related to the experimental period of time selected. In the EFAD rats, cell accumulation was seen initially in the central pulp. This was followed by the sliding of pulp cells located in the central coronal pulp, migrating toward the lateral subodontoblastic area. Near this limit, pulpoblasts were subjected to apoptosis [9]. Dendritic cells and macrophages seem to be implicated in the destruction of pulp fibroblasts. Apoptotic bodies were engulfed by macrophages and destroyed inside lysosomes in an outer subodontoblastic region. This pointed to equilibrium between the generation of new pulpoblasts emerging in the central pulp and the cell destruction occurring mostly in the outer pulp border (Figs. 2.12 and 2.13).
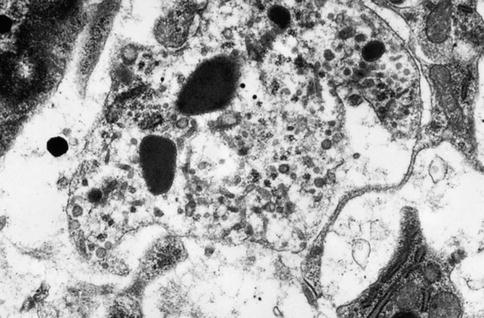
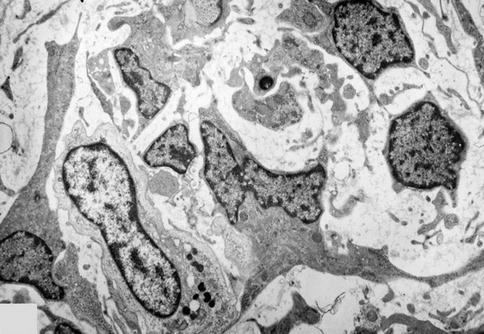

Fig. 2.12
Histiocyte/macrophage pulp cell displaying tubulovesicular structures and electron-dense lysosomes

Fig. 2.13
Heterogeneous cell population in the dental pulp. In addition to fibroblasts, macrophages are seen, loaded with electron-dense lysosomes. Many apoptotic bodies are present in the intercellular spaces
Human dental pulp cells display pluripotency. BMP-2 treatment produced alkaline phosphatase in the cells, which produce and secrete osteocalcin in the culture medium. They also overexpress Sox2, Col2, and ColX. They express two adipocyte-specific transcripts PPARg2 and LPL. Koyama et al. [10] concluded that pluripotent pulp cells differentiate into osteoblasts, chondrocytes, and adipocytes. The dental pulp may provide enough cells for potential clinical applications.
Multipotent and unipotent dental pulp progenitors are currently detected among pulp stem cells. Three clonal pulp precursor lines were established from embryonic ED18 first molars of mouse transgenic for a recombinant plasmid adeno-SV40. The clones were cultured on 2D and 3D scaffolds. They were induced to differentiate toward the odontogenic/osteogenic, chondrogenic, or adipogenic lineage. The A4 clone has the capacity to be recruited toward at least three mesodermal lineages, contributing to dentin-like or bone formation. The A4 lines appeared to be multipotent cells. In contrast C5 and H8 displayed a more restricted potential and were monopotent. In the dental pulp, progenitor cells demonstrate the coexistence of multipotent cells and cells with restricted lineages [11]. The A4 cells express DMP-1 after 4 days of culture, reaching a maximum at 7 days. Then, the expression of the protein was decreased. Fourteen days are needed for A4 cells to reach a maximal expression of DSPP.
2.4.2 Intracellular Proteins
In addition to the series of constitutive proteins, found in most cellular compartments, some intracellular molecules seem to be more specifically expressed by the stromal pulp fibroblasts. They are implicated both in cell differentiation, in the synthesis of ECM molecules, and after endocytosis, in their degradation by MMPs (collagenases, MMP-3, MMP-2, and MMP-9). Pulp cells are also involved in ECM lipids endocytosis [12] (Figs. 2.14a, b, 2.15, and 2.16a, b).
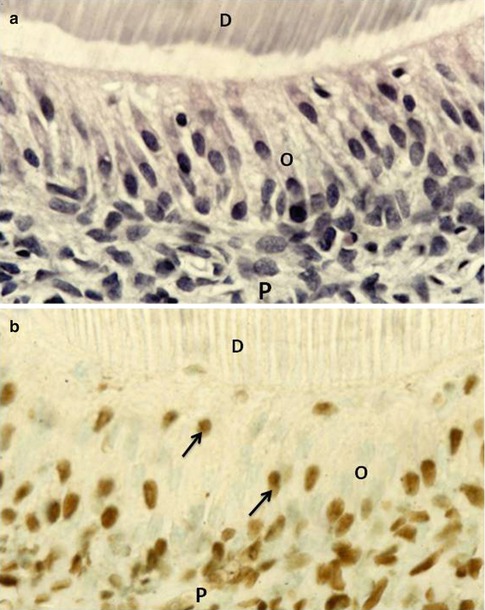
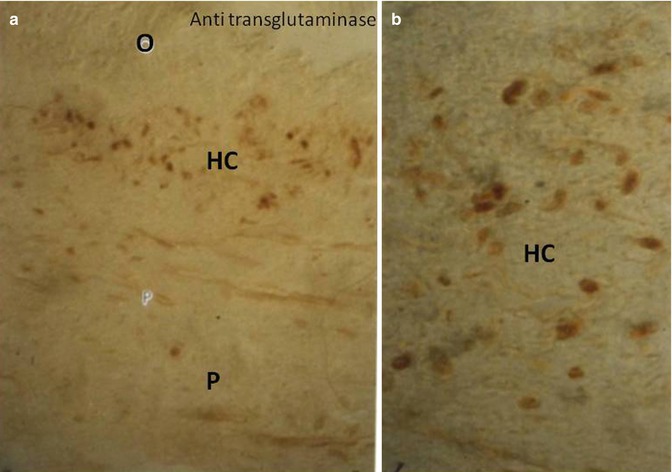
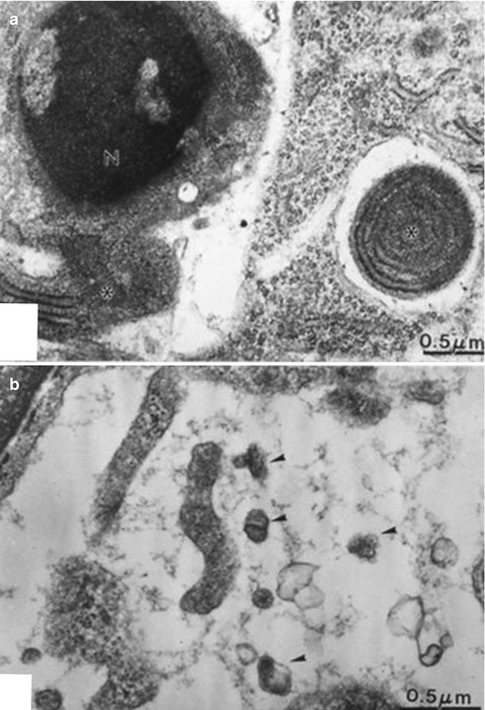

Fig. 2.14
(a) Hematoxylin/eosin staining of a section including the inner part of dentin (D), odontoblasts (O) cell bodies, and pulp (P). (b) Staining the section with the TUNEL method allows visualization of a few apoptotic cells (arrow) within the odontoblast layer, and many cells in the so-called Hoehl’s cell layer above the pulp

Fig. 2.15
(a, b) Immunostaining with an anti-transglutaminase labels mostly the cells located beneath the subodontoblastic Hoehl’s cell layer (HC). O odontoblasts, P pulp

Fig. 2.16
(a) Apoptotic cell with marginal chromatin concentration in the nucleus (N). A cell-derived fragment including rough endoplasmic reticulum is degraded in an electron-lucent vesicle. In (b) apoptotic bodies are seen in the intercellular space (arrowheads)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


