Once differentiation of the cells forming the future face has been initiated by one or more epithelial-mesenchymal interactions, other factors regulate subsequent development and growth of the maxillofacial complex. Individual elements or regions do not develop and grow at the same rate; there is a well-known phenomenon of relative growth. Such regional differences imply that development and growth are not regulated globally.
Control of development and growth is therefore not global but local. Two sets of local controlling factors that can be at play are (1) intrinsic differences in different regions of the skeleton, muscles, nerves and (2) differences in the local environment in which individual elements develop. Evidence for intrinsic differences comes from documentation of differences in osteogenic and hematopoietic capability between bones that develop endochondrally and those that develop intramembranously or differences in inherent versus evoked growth potential (eg, capability of primary cartilages, such as Meckel’s cartilage, to proliferate and grow independently of external influences versus the requirement of secondary cartilages, such as that of the condylar process of the dentary, for extrinsic [muscular] stimulation of growth).
Evidence for differences in local environments within the embryo comes from the concept of functional matrices developed by Moss . During late embryonic life, the development and growth of the cranium are influenced by the expanding and growing brain, those of the mandible and palate by the growing tongue, and those of the maxillofacial region by the expanding eye and growing nasal septum. The same elements of the head and face are therefore subject to regulation by different factors at different times during pre- and postnatal development.
Regulation of the development and growth of individual elements is not a case of all intrinsic or all extrinsic control. These responses are possible because the progenitor cells modulate their proliferative potential in response to stimuli (presumably mechanical) associated with muscle pull. A low intrinsic growth potential can be massively augmented and regulated in response to functional demand, a requirement that can be taken advantage of by surgical manipulation.
Adjacent skeletal units, even though controlled by different functional matrices, influence one another because they are adjacent, begin their development at different times, develop and grow at different rates, and respond to different controls. Thus, the cranium grows fast and early to keep pace with the early and rapidly growing brain, whereas the mandible and maxilla grow slowly and late, with their rates being more in tune with general somatic growth than with brain growth.The influence of cranial growth on facial growth is because of timing, structure, and function. Because the cranium grows to keep pace with the rapid and early growth of the brain, it especially influences growth of the upper third of the face, which, accordingly, displays the fastest rate of facial growth and complete growth first at around 12 years of age. The remainder of the maxillofacial region is less affected by brain growth and, accordingly, grows more slowly and for a longer time, with growth not being completed until the late teens or early 20s when the wisdom teeth erupt.
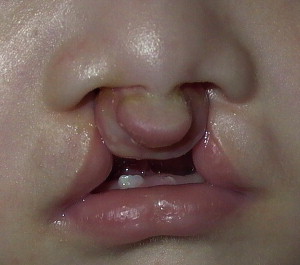
The anterior attachment of the facial skeleton to the base of the skull provides the structural component linking cranial and facial growth. The functional component is provided by the three major sense organs (eye, ear, and nose), with their tissues and cavities acting as functional matrices for maxillofacial growth. Skeletal units have been used to identify the elements of the maxilla that respond by growth to particular functional matrices: growth of the eye influencing the orbital unit, growth of the nasal septal cartilage influencing the nasal unit, growth of the teeth in the alveolar unit, and growth of the maxillary sinus in the pneumatic unit. Progressively, the relevant muscles exert their influence on growth and maintenance of adult form. Thus, the major functions of neural integration—sight, sound, smell, digestion, and respiration—all influence maxillofacial growth to varying degree and at various times during pre- and postnatal development. This explains, in part, the bewildering diversity that is seen clinically, particularly with respect to bilateral cleft lip/palate ( Fig. 1 ).
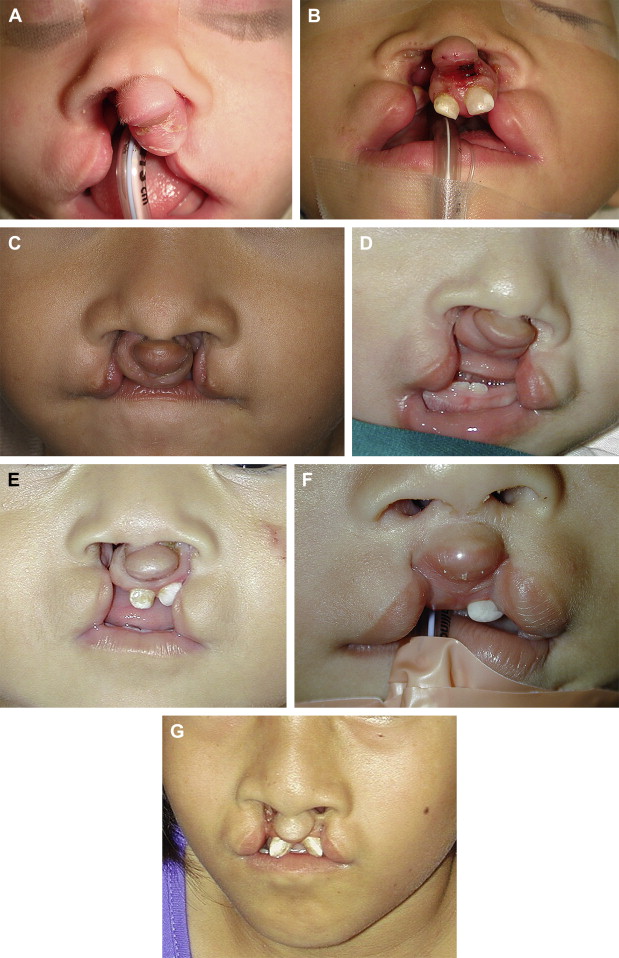
It is evident from the preceding sections that craniofacial development is hierarchic and integrated, with important temporal and spatial components adding to the complexity of control mechanisms. Consequently, there is no single basis for craniofacial malformations. Also to be considered is the remarkable capacity of the embryo to compensate for loss, deficiencies, or deviations from normal development, a phenomenon known as developmental regulation. For example, a halving of the rate of cell division (by slowing the cell cycle) in a population of migrating neural crest cells would be expected to produce a mesenchymal condensation that was only 50% of the normal size (as happens in some mutants). Because osteogenesis may not commence in condensations that are lower than a threshold size, such a reduced rate of cell division would be expected to lead to skeletal deficiencies or abnormalities. An increase in the proportion of cells that are mitotically active (normally, not all the cells in a population are dividing) could compensate for the extended cell cycle, however, resulting in production of a condensation of normal size in which normal skeletogenesis is initiated. Indeed, whole mammalian embryos can compensate for massive reductions in cell number and return to normal size within as little as 3 days. It can therefore be difficult and perhaps misleading to pinpoint alterations in a single development event as the basis for a malformation without a careful analysis of many developmental processes.
Congenital labiomaxillary clefts result from the absence of fusion or incomplete fusion of the maxillary and medial nasal processes. The superficial muscles of the face, which arise from the second branchial arch, migrate laterally and medially between the epidermis and subjacent ectomesenchyme and normally reach the midline in the week that follows fusion of the facial processes. In a complete labiomaxillary cleft, the muscles of the nasal floor and upper lip cannot bridge the gap of the cleft nor can they unite with their muscular counterparts on the noncleft side. The muscular integrity of the region is considerably disrupted, which has a profound effect on the underlying skeleton ( Fig. 2 ).
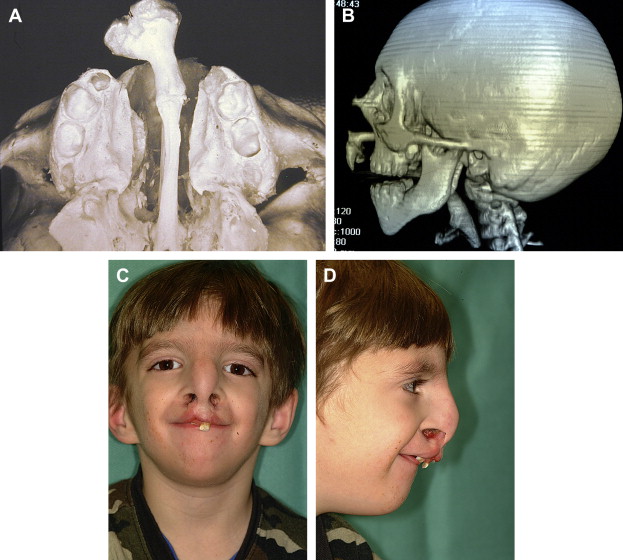
In bilateral clefts, on each lateral side of the labial cleft, the mucocutaneous lining essentially has similar characteristics to those in the total unilateral cleft. On the median process, however, it is different. The skin is actually much retracted and supported by underlying connective tissue that is not retracted. Moreover, the ensemble of skin and connective tissue is compressed by the dental buds, themselves anteriorly displaced by the posteroanterior pressure that is exerted by the lower lip and absence of the external upper labial sling. The columella is also retracted, and its lower part intrudes onto the median process, where it more or less blends in with the skin of the lip ( Fig. 3 ). Other than in exceptional cases of holoprosencephaly, there is little or no true hypoplasia. Simple postoperative distention of the labial skin and columella is therefore, by itself, capable of giving the reconstructed lip good height without having to resort to procedures of elongation that needlessly mutilate the lip. There is no trace of mucocutaneous roll on the median process because its constituent elements, such as the labial muscles, remain on each side of the labial cleft.