7Prevention of Biological Complications
Prevention of biological complications involves identification and management of modifiable risk factors. The following patient-related factors are associated with an increased risk for peri-implant infection:
•Poor oral hygiene
•Tobacco smoking
•History of treated periodontitis
•Presence of periodontal pockets (≥ 5 mm with concomitant bleeding on probing)
•Lack of supportive or maintenance care
•Diabetes mellitus (has also been suggested as a risk factor, although poor control of the diabetes may be of greater importance)
Site-related factors associated with peri-implant infection include:
•Insufficient bone volume at the implant recipient site
•Incorrect three dimensional implant position
•Proximity of implants/teeth
•Insufficient width of keratinized peri-implant mucosa
For patients presenting with multiple risk factors, placement of implants should be delayed until all factors have been addressed. For example, in a patient with periodontitis with poor oral hygiene who smokes 20 cigarettes per day, the risks are expected to be much greater than in a patient who has only one risk factor such as tobacco smoking. Implant placement should only be performed when the periodontal health is stable, the patient’s oral hygiene has improved (full-mouth plaque score/FMPS less than 20%), and smoking cessation advice has been provided.
The following stages of the implant treatment process play an important role in the prevention of future biological complications:
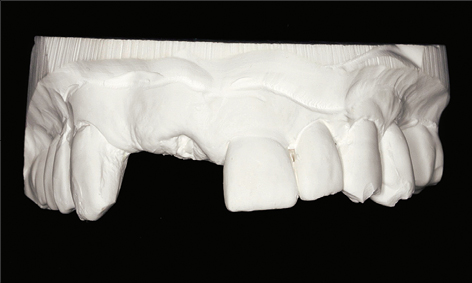
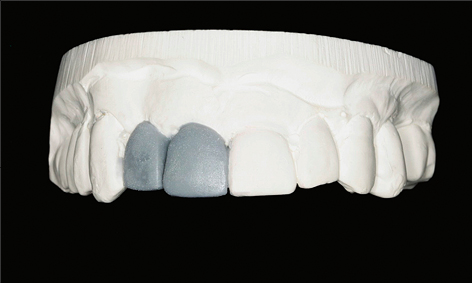
As part of the treatment planning stage, a careful assessment of the patient’s requirements and expectations, a thorough medical and dental history, and a comprehensive clinical examination are required. The use of a diagnostic wax-up, a radiographic stent, and appropriate radiographs are important in this planning stage (Figs 1a–c). These steps will ensure that potential patient- or site-related risk factors for future biological complications are identified and discussed with the patient prior to treatment commencing.
7.1.2Preparation of the Patient
In patients diagnosed with chronic or aggressive periodontitis, completion of active periodontal therapy, aiming for elimination of residual pockets with bleeding on probing, should precede implant placement.
In patients with a history of treated periodontitis with residual probing depths (PD) at least 5 mm with concomitant bleeding on probing, FMPS of more than 20% and associated risk factors, retreatment, and periodontal reevaluation are recommended before implant placement. The increased risk for peri-implant infection should be discussed with patients who have a history of periodontitis prior to implant treatment. A maintenance care program with shorter intervals should be planned for patients diagnosed with aggressive periodontitis.
Tobacco smokers should be informed of their increased risk of peri-implant infection and provided with smoking cessation advice.
7.1.3Preparation of the Recipient Site
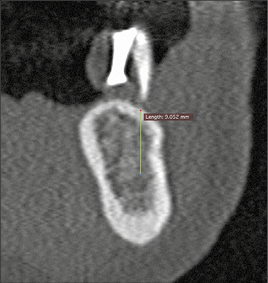
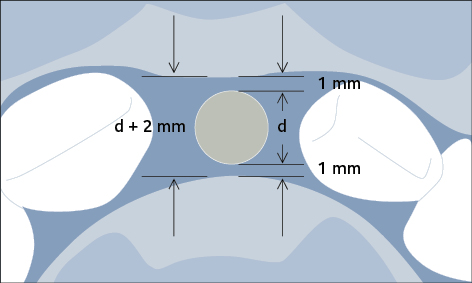
Preparation of the recipient site may involve hard- or soft-tissue grafting procedures, to ensure an adequate volume of bone and width of keratinized mucosa prior to implant placement. If bone augmentation is required, a staged or simultaneous approach may be chosen depending on the available bone volume for correct three-dimensional implant positioning. A minimum of 1 mm of bone surrounding the entire implant circumference is required to avoid incomplete osseointegration of the implant, which may result in subsequent biological complications (Fig 2). The appropriate choice of the implant diameter is therefore important to prevent future biological complications.
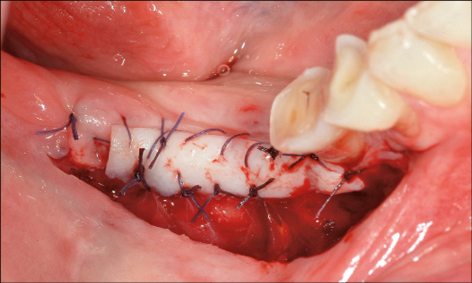
The effect of the width of the keratinized mucosa on peri-implant health outcomes may be influenced by a number of factors, including the patient’s oral hygiene, and on the supportive post-implant therapy provided (Frisch and coworkers 2014). Nevertheless, a recent systematic review addressing the necessity of keratinized mucosa for peri-implant health concluded that an adequate zone of keratinized mucosa (approx. 2 mm) is related to better peri-implant health (Brito and coworkers 2014). Furthermore, in the completely edentulous mandible an insufficient band of keratinized mucosa (less than 6 mm) on the orofacial aspect was associated with a higher incidence of biological complications in the first year following provision of full-arch implant-supported mandibular prostheses (Maló and coworkers 2013). Therefore, establishing an adequate band of keratinized tissue can be considered as a preventive measure for future biological complications (Figs 3a–c). Where keratinized mucosa is minimal or absent, soft-tissue grafting may be performed either prior to implant placement, at the time of second-stage surgery if submerged healing has occurred, or following restoration of the implant as a preventive measure.
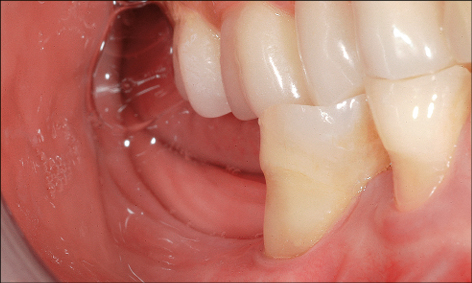
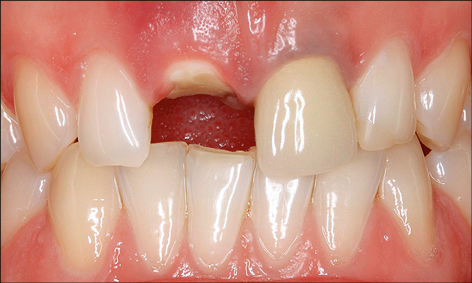
Preparation of the Implant recipient site may also involve orthodontic treatment to create the ideal space for implant placement and subsequent restoration. Orthodontic tooth extrusion may also be considered in situations where the crown has fractured subgingivally, in order to optimize the bone and soft-tissue volume at the implant recipient site (Fig 4).
A correct surgical technique (sufficient cooling, minimal pressure, sharp drills) during osteotomy preparation is important (Buser et al. 2004) to prevent overheating of the alveolar bone, which would result in marginal bone loss and incomplete osseointegration of the implant.
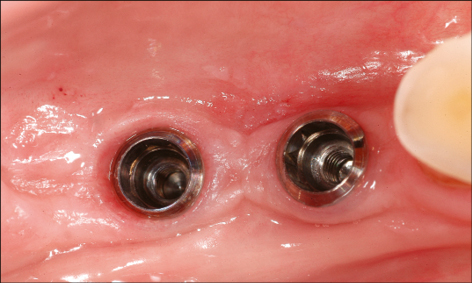
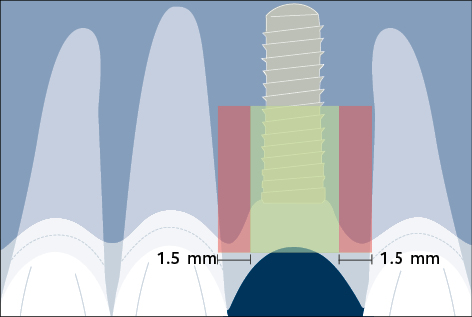
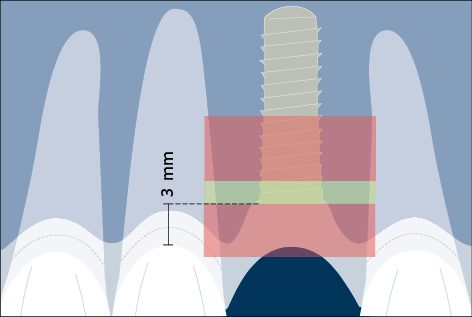
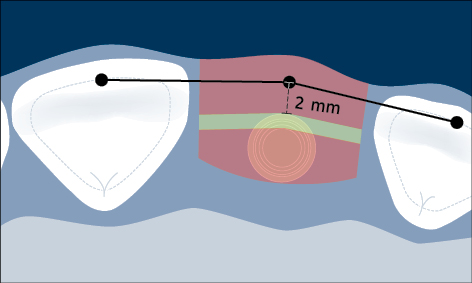
Correct three-dimensional positioning of an implant is important to prevent risk of biological and esthetic complications (Figs 5a–c). Placement of implants too close together (less than 3 mm between adjacent implants), or too close to neighboring teeth (distance from implant platform to tooth less than 1.5 mm) may result in insufficient access for adequate plaque control and subsequent peri-implant infection (Serino and Ström 2009; Abi Nader and coworkers 2014).
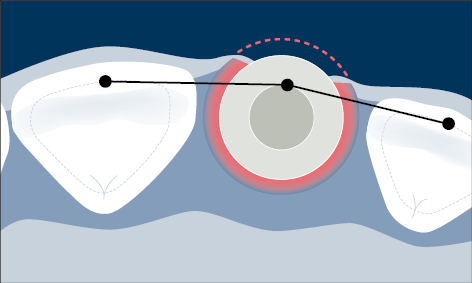
Malpositioned implants may result in marginal bone loss, mucosal recession, and exposure of part of the implant surface to the oral cavity, which enables a biofilm to readily form on the endosseous portion of the implant with the risk of peri-implant infection (Fig 6).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses