Introduction
The aims of this study were to evaluate with microcomputed tomography the orthodontically induced inflammatory root resorption in premolars caused by buccopalatal jiggling movement with light and heavy forces and to compare it with the resorption caused by equivalent but continuous buccal forces.
Methods
The sample consisted of 60 maxillary first premolars collected from 30 patients (15 girls, 15 boys; ages, 13-18 years) who required orthodontic treatment with extractions. They were divided into 3 groups of 10 patients. Light (25 g) or heavy (225 g) buccal tipping orthodontic forces were randomly assigned on the maxillary right or left quadrant with either continuous buccal (positive controls) or buccopalatal jiggling forces for 12 weeks. At the end of the experimental period, the teeth were carefully extracted and processed for 3-dimensional imaging and volumetric evaluations of resorption craters. Data were analyzed with Wilcoxon signed rank tests.
Results
There was no statistically significant difference between positive control light ( P = 0.0173) and heavy ( P = 0.0173) continuous forces and jiggling forces for both force magnitudes. However, statistically significant differences were observed between heavy and light jiggling forces ( P = 0.038), with heavy jiggling forces causing greater total root resorption than light jiggling forces.
Conclusions
Light and heavy jiggling forces in the buccopalatal direction did not cause significantly different amounts of root resorption when compared with continuous forces of the same magnitude. On the other hand, light jiggling forces resulted in less root resorption than heavy jiggling forces.
Highlights
- •
We evaluated root resorption crater volumes with different force applications.
- •
Continuous vs jiggling forces of the same magnitude produce similar volumes of root resorption.
- •
Heavy jiggling forces produce greater root resorption than light jiggling forces.
- •
Information on etiology of root resorption will decrease orthodontic treatment side effects.
Orthodontically induced inflammatory root resorption (OIIRR) is defined as the destruction of formed tooth structure; it is a major side effect of orthodontic treatment. The initial phase of root resorption is associated with orthodontic overcompression of local areas of the periodontal ligament (PDL) that undergo aseptic necrosis, called hyalinization. The first resorption occurs in the periphery of the main necrotic hyalinized zone, beneath the main bulk of necrotic tissue. The initial penetration of cells into precementum and cementum occurs at the peripheries or at a short distance from the peripheries of the hyalinized zone, with tartrate resistant acid phosphatase (TRAP)-positive cells first observed in the bone marrow spaces. During later stages of tissue reactions, the majority of the cells involved in removal of the necrotic tissues and resorption of the root surfaces are multinucleated TRAP-positive cells that, when reaching the subjacent contaminated and damaged root surface after having removed necrotic tissue, continue to remove the cementum surface.
Radiographic evidence of root resorption as a result of orthodontic treatment was first described by Ketcham in 1927. Linge and Linge in 1991 found that 16.5% of orthodontically treated patients demonstrated root shorthening of more than 2.5 mm in at least 1 maxillary incisor. Davidovitch in 1996 reported that 1% to 5% of the treated population experienced excessive root resorption, leading to a loss of more than a third of the initial root length. Thus, OIIRR has great clinical significance during orthodontic treatment.
Although optimal force systems have been advocated to be capable of producing a maximum rate of tooth movement without tissue damage and with maximum patient comfort, the optimal force for tooth movement may differ for each tooth and for each patient. Both patient-related factors and orthodontic treatment–related factors have been reported to contribute to the degree and the severity of OIIRR. Previous investigations have linked the severity of root resorption to various factors, including type of orthodontic appliance, magnitude of applied force, duration of force application, type of tooth movement, clinical factors, systemic factors, patient age, genetic factors related to root anomalies, previous trauma, and ethnicity.
Pretreatment factors were evaluated in the patients’ records to help clinicians predict the incidence, location, and severity of root resorption before the orthodontic treatment. It was reported that resorption occurs primarily in the maxillary anterior teeth, with the worst resorption in the maxillary lateral incisors and in teeth with abnormal root shapes (pipette, pointed, or dilacerated). In addition, patients of Asian origin experience significantly less root resorption than do white patients or those of Hispanic origin; thus, tooth morphology and ethnicity parameters were reported to have significant associations with orthodontic root resorption. In relation to the type of orthodontic treatment, patients who had first premolar extraction therapy had more resorption than those who had no extractions or had only maxillary first premolars removed. Duration of treatment and horizontal (but not vertical) displacement of the incisor apices also affected proportionately root resorption, but no differences were found for slot size, archwire type, elastics, and types of expansion. As far as biomechanical factors of orthodontic forces are concerned, human studies have shown that increased root torque, tip, and rotational movements result in greater root resorption.
The effects of jiggling forces on root resorption were investigated in animal studies. Controversial results were obtained from these studies. Kim and Son found that root resorption patterns were not different between jiggling and unidirectional forces. Nevertheless, the amount of root resorption in rats with jiggling forces was significantly greater but with no significant differences in resorption areas between rats that received light (10 g) and heavier (50 g) forces. The effects of jiggling forces on the extent of root resorption craters in human teeth have not been investigated yet.
The aim of this study was to investigate the presence and dimensions of root resorption craters in premolars after the application of buccopalatal jiggling movements vs continuous buccal movements with light and heavy forces for 12 weeks using microcomputed tomography scans. In other words, we estimated how the application of buccopalatal jiggling movements with light and heavy forces affected root surfaces and how the quantitative properties of the resorption craters were altered compared with equal forces continuously applied in the same buccal direction. The significant aspects of this study were to determine whether different jiggling force levels have different adverse effects on root resorption and to quantify the resorption craters. Our team has already conducted several studies evaluating OIIRR caused by varying degrees and types of force application with microcomputed tomography and its associated computer software, which enables the volume of root resorption craters to be measured and the effects to be quantified. However, the impact of jiggling movements on teeth has never been looked at before.
Material and methods
The sample consisted of 60 maxillary first premolars collected from 30 patients (15 girls, 15 boys) who required orthodontic treatment with extractions. Their ages ranged from 13 to 18 years. The selection criteria were (1) no previous reported or observed dental treatment to the teeth to be extracted, (2) no previous reported or observed trauma to the teeth to be extracted, (3) no previous reported orthodontic treatment involving the teeth to be extracted, (4) no past and present signs and symptoms of periodontal disease, (5) no signs and symptoms of bruxism, (6) medical history free of any significant conditions that would affect the dentition, (7) completed apexogenesis, (8) knowledge of the subject’s residential status since birth in an area with unfluoridated water, (9) normal craniofacial and dentoalveolar developmental anatomy, and (10) sufficient room for the proposed root movements to be carried out.
The patients were divided into 3 groups of 10 each. More precisely, they were grouped as follows: (1) positive control heavy and heavy jiggling movement group: these patients had their maxillary right or left first premolars randomly selected and moved only buccally for 4 weeks with 225 g of heavy force and the opposite first premolar moved buccally for 4 weeks, then palatally for the next 4 weeks, and finally buccally again for the last 4 weeks with 225 g of heavy forces; (2) positive control light and light jiggling movement group: these patients had their maxillary right or left first premolars randomly selected and moved only buccally for 4 weeks with 25 g of light force, and the opposite first premolar moved buccally for 4 weeks, then palatally for the next 4 weeks, and finally buccally again for the last 4 weeks with 25 g of light forces; and (3) light and heavy jiggling movement group: these patients had their maxillary right or left first premolars randomly selected and moved buccally for 4 weeks, then palatally for the next 4 weeks, and finally buccally again for the last 4 weeks with 225 g of heavy force on one side and 25 g of light force on the opposite side ( Fig 1 ).
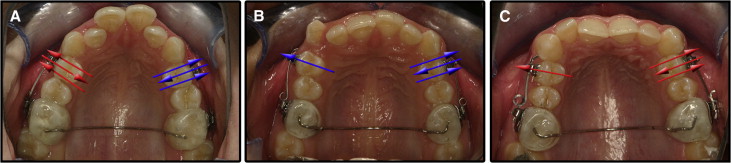
Written and verbal informed consent was acquired from the subjects and their parents or guardians. Ethics approval was obtained from Bülent Ecevit University (approval no. 2012/06) in Zonguldak, Turkey.
Orthodontic brackets (SPEED; Strite Industries, Cambridge, Ontario, Canada) were bonded to the maxillary first molars and first premolars. Sectional archwires were constructed to generate buccal only (positive controls) or buccal and palatal (jiggling) movements. Beta-titanium-molybdenum alloy wires (0.017 × 0.025 in) were applied in the heavy force groups and measured to generate 225 g of force, and beta-titanium-molybdenum alloy wires (0.016 in) that generated 25 g of light force were used in the light force groups. Transpalatal arches of 0.9-mm stainless steel wires were embedded in acrylic and fitted to the maxillary molars in the form of blocks. In this way, stabilization of the molars and opening of the occlusion were achieved to allow the teeth to move freely once the forces were applied ( Fig 1 ). One operator (F.C.) treated all patients; at the end of the 12-week experimental period, the first premolars were extracted by the same surgeon under a strict protocol that ensured no surgical trauma to the root cementum. After the extractions, the teeth were stored in individual containers of sterilized deionized water (Milli-Q; Millipore, Bedford, Mass), which is considered the appropriate storage medium according to a previous study. An ultrasonic bath for 10 minutes followed by a mechanical rubbing motion with a damp gauze swab resulted in removal of all traces of residual PDL and soft tissue fragments. The specimens were disinfected by immersion in 70% alcohol for 30 minutes, with final bench drying for at least 48 hours.
In 1973, Hounsfield developed a commercial system for medical imaging. This was a significant step forward in diagnostic medicine because it allowed images of internal features to be made based directly on their x-ray attenuation coefficients. Nevertheless, current technology limits the use of microcomputed tomography to small, inanimate specimens, such as bone and extracted tooth samples. The nondestructive nature of the technique allows a variety of experiments to be conducted to investigate structural and compositional characteristics of calcified tissues.
The scanning and measurement of the resorption craters in the sample were conducted with a SkyScan 1172 desktop x-ray microtomograph (SkyScan, Aartselaar, Belgium). The SkyScan 1172 is a compact desktop system for microscopy and microtomography. It consists of an x-ray shadow microscopic system and a computer with tomographic reconstruction software. The system allows a nondestructive 3-dimensional (3D) reconstruction of the object’s inner structure from 2-dimensional x-ray shadow projections. Multiple x-ray shadow transmission images of the object from different angular views are obtained as the object rotates on a high-precision stage. From these shadow images, cross-sectional images of the object are reconstructed by a modified Feldkamp cone-beam algorithm, creating a complete 3D representation of the internal microstructure and the density over a selected range of heights in the transmission image. After the serial construction, the cross sections were displayed as well as a realistic 3D image with possibilities to rotate and cut the object model.
Each crater was isolated, and the volume was measured with the convex hull software program, developed by the Australian Centre for Microscopy and Microanalysis at the University of Sydney; it detects interruptions of the tooth surface, connects the borders of lacunae, and applies 2-dimensional convex hull algorithms to each axial slice.
Results
After the 3D reconstructions of the root images, each resorption crater was isolated; x, y, and z coordinates were recorded, and the total resorption for each premolar specimen was calculated according to the sum of all craters on each tooth.
Statistical analysis
Descriptive statistics and statistical analyses were performed using the Statistical Package for Social Sciences (version 20 for Windows; IBM, Armonk, NY). The means, standard deviations, and ranges of root resorption crater volumes were calculated. The Wilcoxon signed rank test was applied to assess the statistical differences between the same magnitudes of light buccopalatal jiggling force to light continuous buccal force, heavy buccopalatal jiggling force to heavy continuous buccal force, and light buccopalatal jiggling force to heavy buccopalatal jiggling force in the relevant patient groups. P ≤ 0.05 was considered to be significant.
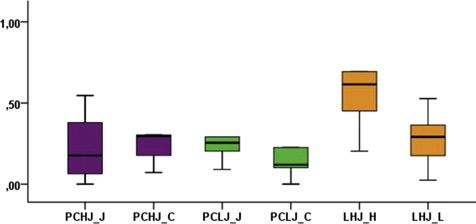
There was no statistically significant difference between the heavy continuous buccal and heavy buccopalatal jiggling forces ( P = 0.173), with the corresponding mean values of resorption crater volumes in each group estimated at 0.355 and 0.278 mm 3 , respectively. The amounts of root resorption were 0.195 mm 3 caused by light continuous buccal forces and 0.291 mm 3 caused by light buccopalatal jiggling forces, with no statistical significance between those values ( P = 0.173). When calculating root resorption in the groups that received only buccopalatal jiggling forces, light and heavy forces resulted in means of 0.265 and 0.710 mm 3 of root resorption, respectively, and the difference between them was statistically significant ( P = 0.038) ( Table , Fig 2 ).
| Group | Minimum | Maximum | Mean ∗ | SE | SD | P value |
|---|---|---|---|---|---|---|
| PCHJ | ||||||
| Jiggling | 0.00001 | 0.92522 | 0.278 | 0.09966627 | 0.29899882 | 0.173 |
| Control | 0.071600 | 0.939430 | 0.355 | 0.098350320 | 0.295050961 | |
| PCLJ | ||||||
| Jiggling | 0.09128 | 0.59943 | 0.291 | 0.05937041 | 0.17811123 | 0.173 |
| Control | 0.00000 | 0.49993 | 0.195 | 0.06006714 | 0.18020141 | |
| LHJ (heavy jiggling force) | 0.20333 | 1.79757 | 0.710 | 0.17403291 | 0.52209872 | 0.038 |
| LHJ (light jiggling force) | 0.02496 | 0.52712 | 0.265 | 0.05677588 | 0.17032764 | |
Discussion
External apical root resorption is a common phenomenon associated with orthodontic treatment. The factors relevant to root resorption can be divided into biological and mechanical, with some of them associated with an increased risk of root resorption during orthodontic treatment. As far as mechanical factors are concerned, extensive tooth movement, type of movement (eg, root torque and intrusion), and magnitude and duration of orthodontic forces have been highly correlated to root resorption.
There is no consensus, nor are there other clinical studies regarding, specific evidence of how jiggling forces can affect the pattern and amount of root resorption. In this study, we investigated how the application of buccopalatal jiggling movement with light and heavy forces affected root surfaces and how the quantitative properties of resorption craters were altered, thus determining whether different buccopalatal jiggling force levels have different adverse effects on root resorption and quantified resorption craters. The effects of jiggling force on root resorption were investigated in animal studies, and controversial results were obtained. Kim and Son found that in the cat model, although alveolar resorption was more severe by the application of jiggling forces than by unidirectional forces, root resorption patterns were not different between jiggling and unidirectional forces; this agrees with our results. However, in a rat model, the amount of root resorption and the number of resorptive TRAP-positive cells were significantly greater in rats that received multidirectional and alternating forces at short intervals of jiggling compared with rats having the force directions changed at longer intervals. Similar to our study, in the rat model, different force levels of light (10 g) and heavy (50 g) forces did not produce significantly different amounts of root resorption. Thus, the authors concluded that jiggling forces, applied alternately in different directions with a short interval of reactivation, are critically important in creating severe root resorption. It was also pointed out that the parameter of reactivation intervals seemed to influence the levels of root resorption, with short intervals between force reactivation in conjunction with alterations in force direction producing greater root resorption.
Our search of the orthodontic literature showed that the effects of jiggling forces on the extent of root resorption craters in human teeth has not been investigated. Therefore, we attempted to elucidate whether buccopalatal jiggling movements with light and heavy forces for 12 weeks would result in differences in the volumes of resorption craters compared with equivalent but continuous heavy and light forces in the same subjects studied with microcomputed tomography scans. This study was a continuation of a series of investigations conducted by the orthodontic department of the University of Sydney in Australia on orthodontic root resorption.
As already reported in the literature, force magnitude, direction, and duration have been considered significant factors in the etiology of OIIRR. Even though several studies have been performed to define the optimal force system, inconsistencies in research designs resulted in controversial results. In a series of clinical investigations, the associations among duration, magnitude, and type of applied force in relation to the amounts of achieved tooth movement and root resorption were studied. When a continuous force of a clinically relevant magnitude (50 g) was applied, tooth movement increased gradually over time. After 3 weeks, a few teeth showed root resorptions extending halfway to the pulp or more in the apical third of the root. Doubling the force magnitude (100 g) did not affect the tooth movement or the severity of root resorption. However, when the force was increased by 4 times (200 g), tooth movement increased by 50%, but still without a significant increase in the occurrence or severity of root resorption. Tooth movement was achieved more efficiently with a continuous force than with an interrupted force of the same magnitude. Root resorptions, though, did not seem to be affected differently by the 2 types of forces; repair with secondary cementum was recorded almost 3 times more often after longer healing periods. Irrespective of magnitude and type of force, large individual variations were observed regarding tooth movements and root resorptions as well as their reparative potential. On the other hand, different continuous moderate forces were compared with heavy forces to evaluate the amounts of root resorption and tooth movement in the rat model. The initial tooth movement was not proportionally related to different levels of force (10, 25, 50, and 100 g), but after a lapse period, light forces resulted in increased rates of tooth movement and significantly less root resorption compared with heavier forces. This is consistent with similar observations on the effect of continuous forces of different durations and magnitudes in human premolars. Significant differences in the extent of root resorption were found between the different time points and force levels, and the volumes of the root resorption craters were related and increased with longer force applications and higher magnitude levels. In the various animal and human studies, there is clearly disagreement in the research results on the mechanism and extent of tooth resorption. More precisely, previous studies reported that when applying continuous forces, increased force levels seemed to result in increases in root resorption. The same propensity was found in this study when heavy jiggling resulted in greater root resorption than light jiggling. However, our results support the notion that buccopalatal alterations in force direction do not evoke different amounts of resorption phenomena compared with equivalent continuous and unidirectional buccal forces. In other words, when keeping the force magnitude invariable, the tooth movement, with either buccopalatal jiggling or continuous force in the buccal direction, resulted in similar amounts of root resorption.
The response of alveolar bone to jiggling forces has been extensively studied. Observations in animal experiments have implicated jiggling forces in the etiology of infrabone defects. The most significant finding in this regard was that jiggling forces in a dog model enhanced the rate of periodontal destruction in teeth that were also subjected to ligature-induced and plaque-associated marginal periodontitis. When jiggling forces were applied to established marginal periodontitis, increased bone resorption and loss of alveolar bone were observed. The elimination of trauma on teeth with marginal inflammation did not reduce tooth mobility or increase bone volume. Osseous regeneration and decreased tooth mobility occurred after resolving both components; however, similar findings occurred after resolving inflammation in the presence of continued tooth mobility. After resolution of inflammation, the remaining tooth mobility does not result in increased loss of connective tissue attachment. On a clinical level, the resolution of marginal inflammation is of prime importance in the management of periodontal disease, even with jiggling forces. It can thus be assumed that apart from root resorption, which according to our results was not significantly increased by buccopalatal jiggling forces compared with continuous buccal ones, the importance of adequate control and maintenance of reduced plaque levels need not be underestimated to prevent detrimental effects of jiggling forces on the periodontium.
During orthodontic treatments, reactivations of the appliances are carried out to achieve the desired tooth movements. To clarify whether continuous or intermittent forces of the same direction, reactivated either every 2 or every 3 weeks, produced different levels of tooth resorption, Aras et al reported that even though continuous forces resulted in significantly greater tooth movement, the detrimental effects on root resorption outmatched this result. Intermittent forces caused less root resorption than continuous forces, and root resorption decreased irrespective of the timing of reactivation, when a pause was given. With respect to the results of our study, similar findings were obtained for jiggling forces and buccopalatal alterations in force direction in a weekly interval. Since intermittent forces resulted in less resorption than continuous forces of the same direction because of the pause period, jiggling forces of alternating buccopalatal directions in this study converged to the same results, thus indicating that alterations in direction might counteract continuous resorptive phenomena produced by continuous unidirectional buccal forces. This agrees with an animal study that evaluated tissue responses on a cellular level. It was found that after appliance reactivation during the peak of osteoclastic stimulation, a second cohort of osteoclasts can be recruited, but only after a delay of several days. In addition, appliance reactivation that followed decay of the first activation produced efficient tooth movement without an increased risk of root resorption, since these changes were not accompanied by rapid osteoclast recruitment at compression sites. The authors concluded that timing reactivations for the latter portion of the previous bone remodeling cycle could have significant clinical advantages in promoting tooth movement and reducing the incidence of root resorption. A more relevant study, in terms of clinical application, on extraoral traction for anchorage and its jiggling effect on anchor teeth showed that nighttime use of extraoral traction for a 6-month period resulted in similar degrees of root resorption of the maxillary molars as those in which anchorage was given by a Goshgarian bar or Class II elastics, thus designating once more the possible root healing effect of pauses in force reactivation.
Finite element analysis has proved that when a force is applied on a tooth root, this force is perceived and translated as stress on the PDL. As a result, the actual stress distribution pattern within the PDL plays an important role in the initiation of alveolar remodeling and resultant bone or root resorption. Because of the stress distribution pattern within the PDL in conjunction with the results on osteoclastic recruitment and cell cycle, it may be indicated that certain time intervals are required for the cells to perceive stress alterations and respond with osteoclast activation and resorptive activity. Consecutively, buccopalatal alterations in force direction, as produced in this study by jiggling forces, could result in alternating loading and unloading conditions within the PDL, thus compensating for the destructive effects of continuous unidirectional forces in bone and subsequent root resorption.
Apart from mechanical factors related to force characteristics, the effect of genotype on susceptibility or resistance to the development of root resorption secondary to orthodontic force has been studied. In a mouse model, different inbred strains were used, and the results showed that certain species were highly susceptible to root resorption, whereas others were much more resistant, thus supporting the hypothesis that susceptibility or resistance to root resorption associated with orthodontic force is a genetically influenced trait. Human studies relating the vitamin D receptor Taql polymorphism with external apical root resorption found that genotypes containing the C allele were weakly associated with protection against external apical root resorption in orthodontic patients. Sib-pair linkage and parents-child trio association studies found that genetic factors play marked roles in OIIRR, accounting for half to two thirds of the interindividual variations. Similar results were obtained, with significant statistical differences among the frequencies of the alleles and genotypes of the IL-1beta gene polymorphism between the affected and unaffected groups, suggesting that the polymorphism of the IL-1beta gene is associated with root resorption in the studied population.
Orthodontic jiggling forces that were clinically introduced in our study with alterations in the buccopalatal force direction every 4 weeks are relevant to reactivation intervals used in everyday clinical orthodontic practices and did not result in greater root resorption levels than continuous buccal forces of the same magnitude. During the initial phases of leveling and alignment of the dental arches, sequential nickel-titanium wires of increasing dimensions are usually applied, leading to buccal expansion, especially in the intercanine and interpremolar areas. The application of these preformed nickel-titanium archwires has not been found to emulate the natural human arch form, but later placement of stainless steel archwires for dental arch coordination and restoration of a more natural size and form leads to longer treatments and “round tripping” of teeth. It has been hypothesized that these alterations in force direction could result in deleterious tissue effects. These results correlate to our study only for heavy jiggling forces, thus indicating that when light forces are applied, root resorption cannot be attributed to “round tripping” of teeth but rather to extremely high force levels. Even when different biomechanical systems were compared, as when tooth retraction after premolar extraction was performed with either continuous or sectional arch mechanics, the same levels of resorption were found irrespective of the biomechanical system used.
Overall, the side effects of root resorption may be due not only to “round tripping” of teeth or force-related mechanical factors, but also to interindividual patient variations and susceptibilities. This necessitates future studies focused on the development of individualized biomechanical force systems according and related to the patient’s tissue tolerance thresholds, thus preventing root resorption and promoting normal tissue remodeling and healing.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


