Introduction
The force application period is a modifiable factor in root resorption. There is still ambiguity if the continuity of force application is advantageous in terms of root resorption and tooth movement. In this prospective randomized clinical trial, we compared the effects of 2 reactivation periods of controlled-intermittent and continuous forces on root resorption and tooth movement.
Methods
Thirty-two patients were randomly divided into 2 groups: 2 weekly and 3 weekly reactivations. A split-mouth setup was used for the intermittent and continuous force comparisons. The intermittent force was designed with a pause of 3 days before each reactivation of the springs. A buccally directed tipping force (150 g) was generated with 0.017 × 0.025-in Beta III Titanium cantilever springs (3M Unitek, Monrovia, Calif). After the extractions, surface analysis was performed with microcomputed tomography (model 1172; SkyScan, Aartselaar, Belgium) and specially designed software (CHull2D) for direct volumetric analysis. Buccal premolar movement was also measured on the images of the study casts.
Results
Continuous forces produced more resorption than intermittent forces on the total volumes in both groups. A significant difference was found for the 3-weekly group only ( P <0.01) on the cervical-mesial ( P <0.01) and cervical-buccal ( P <0.05) compression regions. In the 2-weekly group, differences were evident in the middle-distal ( P <0.05) and middle-lingual ( P <0.05) tension regions. Continuous forces produced significantly more tooth movement than did the intermittent forces for both the 2-weekly ( P <0.01) and the 3-weekly ( P <0.001) regimens. Significant differences were not observed between the 2 intermittent force regimens regarding root resorption and tooth movement.
Conclusions
Intermittent force causes less root resorption and tooth movement than continuous force. Root resorption decreases irrespective of the timing of reactivation, when a pause is given. On the other hand, timing of reactivation might have critical importance on continuous force applications, since 2 weekly reactivations produced faster tooth movement with similar root resorption when compared with intermittent force.
Root resorption is an inevitable side effect of orthodontic tooth movement. Although the exact etiology has yet to be determined, factors including the magnitude of applied force and the duration of force application have been shown to influence the amount of root resorption. A pause in tooth movement allows time for cemental repair, and many authors believe that discontinuous forces cause less resorption. Even though continuous forces are more effective for tooth movement, root resorption increases as long as the force application extends. The period of force application for minimal root resorption with effective orthodontic tooth movement is the most important question to be answered.
Several studies with different methods have been carried out to find an optimal balance between modifiable factors, such as force magnitude, type, and period. A conclusion has not yet been made.
Two radiographic studies indicated that removable appliances are safer in terms of apical root resorption on incisors.
In a histologic study, the authors applied 50 cN (about 50 g) of buccal tipping forces for different periods. In 1 group, a single activation of the premolars for 3 weeks with an additional week of recovery served as the interrupted force. In the other group, a single activation of the premolars for 3 weeks was repeated after the recovery period. It was reported that there was no difference between continuous and interrupted forces.
In contrast, the histologic-histomorphometric study by Maltha and Dijkman indicated that discontinuous forces generated less resorption. Similarly, Acar et al found that 100 g of discontinuous (12 hours/day) intrusive forces resulted in less root resorption than continuous (24 hours/day) forces with scanning electron microscope analysis.
It was reported that 2-dimensional evaluation methods such as radiographs and histologic sectioning were deficient in the understanding of this 3-dimensional event. However, the 3-dimensional quantitative evaluation method of root resorption is feasible, highly accurate, and repeatable. Weiland used the confocal laser scanning microscopy method with special software for estimating and calculating 3-dimensional root anatomy. Superelastic nickel-titanium wires (0.016 in) were used as the continuous force source and stainless steel wires as the dissipating force source on 84 premolars with force levels of 0.8 to 1 N for 12 weeks with 4 weekly activations. The author reported that continuous forces led to significantly more root resorption and tooth movement. Continuous and controlled intermittent forces applied for 8 weeks were compared in terms of root resorption volume in a microcomputed tomography study by Ballard et al. A buccally directed force (225 g) was applied on 16 maxillary premolars. The intermittent force period was active for 4 days and passive for 3 days. It was reported that intermittent forces led to less resorption volume ( P <0.05), particularly on the buccal-cervical regions ( P <0.001).
It remains unknown whether there is a threshold time interval that is similar to or different from the continuous force. In this study, we aimed to offer insight into the effects of 2 reactivation periods of intermittent and continuous forces on root resorption and tooth movement with microcomputed tomography.
Material and methods
This study was undertaken on 64 maxillary premolars of 32 subjects who required bilateral extractions of premolars for orthodontic purposes (ethics approval numbers, 2007/146 and 08-2008/10766). Twenty-five female and 7 male patients (mean age, 14.4 years; range, 12-18 years), selected according to the criteria described previously, completed a written informed consent. The patients were randomly divided into 2 main groups (2 weekly [2w] and 3 weekly [3w]) each group consisted of 16 subjects. The patients were not matched for age and sex. As a result, the mean ages and standard deviations in groups 2w (12 girls, 4 boys) and 3w (13 girls, 3 boys) were 14.69 ± 2.55 and 13.90 ± 1.62 years, respectively.
These 2 main groups were determined as 2w and 3w for the different reactivation periods. In both main groups, intermittent force was applied on 1 side (groups 2w-I and 3w-I) and continuous force (groups 2w-C and 3w-C), on the other side of the maxillary arch. The location of the intermittent force was assigned randomly to eliminate any allocation bias. We directly bonded 0.022-in SPEED tubes and brackets (Strite Industries, Cambridge, Ontario, Canada) to the buccal surfaces of the maxillary first premolars and molars. The premolars were subjected to a buccally directed tipping force (150 g) with 0.017 × 0.025-in Beta III Titanium cantilever springs (3M Unitek, Monrovia, Calif). Bonding of the brackets, bending of the springs, and calibration of force with a strain gauge (Dentaurum, Ispringen, Germany) was performed by the same clinician (B.A.). Occlusal stops (Transbond Plus Light Cure Band Adhesive, 3M Unitek) were placed on the occlusal surfaces of the mandibular first molars ( Fig 1 ) to prevent occlusal interferences, allow free buccal tipping of the maxillary first premolars, and minimize spring deformation.
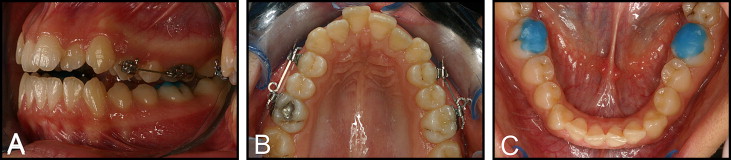
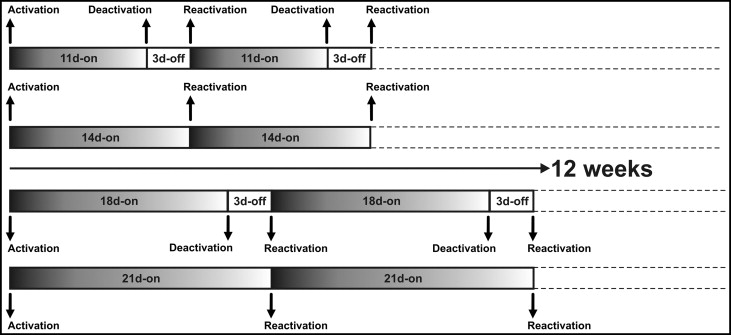
The intermittent force design was based on a pause for the last 3 days of each reactivation period. Eleven and 18 days after the first activations, the cantilever springs were removed, and the teeth rested without force for 3 days on the intermittent sides only. Continuous force was applied for the whole experimental period without any pauses; ie, the springs were not removed on the continuous sides. On the 14th and 21st days, the cycle was restarted with the insertion of the springs on the intermittent sides and the calibration of the springs to the original force level. Concurrently, the springs on the continuous sides were calibrated to 150 g on every 14th and 21st days ( Fig 2 ). Recurrent calibrations of the springs for the intermittent and continuous sides were carried out until the end of the experimental period.
After the 84-day experimental period, the premolars were extracted by the same surgeon with no surgical trauma to the root cementum. The samples were individually stored in Milli-Q water (Millipore, Bedford, Mass). To facilitate the mechanical removal of residual periodontal-ligament and soft-tissue fragments, the samples were subjected to an ultrasonic bath (Transsonic Digitals, Singen, Germany) at 22°C with a 100% vibration level for 20 minutes. The teeth were rubbed with a damp gauze swab, and great attention was paid during this procedure not to cause any damage.
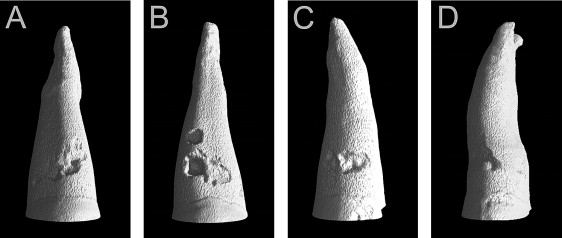
The sample quantification by microcomputed tomography was carried out with the same methodology as described in a previous study, by using a desk-top microtomograph and associated software (model 1172; SkyScan, Aartselaar, Belgium). Only 1 tooth was scanned at a time. All teeth were scanned from the cementoenamel junction to the root apex with resolution between 14.85 and 17.27 μm. After the acquisition, an axial slice-by-slice reconstruction was performed with specific software (NRecon, version 1.4.2; SkyScan). The individual axial 2-dimensional slices were collated to form a 3-dimensional reconstruction of the image by using VG Studio Max software (version 1.2; Volume Graphics, Heidelberg, Germany) ( Fig 3 ). Each resorption crater was isolated and exported to the Convex Hull 2D software (CHull2D, University of Sydney, Sydney, Australia), which made slice-by-slice quantitative volumetric measurements. Assessments of resorption volumes were performed not only for total root surface, but also on 4 surfaces (buccal, lingual, mesial, and distal) and at 3 levels (cervical, middle, and apical).
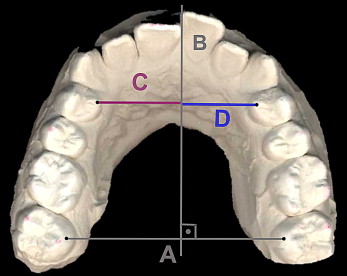
The dental plaster casts of the maxilla, obtained before and 12 weeks after buccal tooth movement, were scanned with a flatbed color image scanner (Expression 1600; Epson, Chatswood, Australia) at 600 dpi, 24-bit gray scale. The acquired images were printed, and transversal guide lines between the mesio-palatinal cusps of the second molars and a perpendicular line were constructed ( Fig 4 ). Distances of the right and left premolars to the perpendicular line were measured with a digital vernier caliper (Masel, Bristol, Pa).
Statistical analysis
The Wilcoxon test was used for the intragroup comparisons of root resorption and tooth movement: ie, the comparisons of root resorption and buccal movement with intermittent and continuous forces. Intergroup comparisons of root resorption and tooth movement were performed with the Mann-Whitney U test.
Results
Of the alterations on the root surfaces in groups 2w-C and 2w-I ( Table I ), the mean total root resorption volumes were 1.01 and 0.75 mm 3 , respectively ( Fig 5 ). Continuous forces showed higher resorption volumes, but the difference between the continuous and intermittent forces was not statistically significant ( P >0.05). Total resorption volumes at different levels and different surfaces also showed no significant differences between the groups ( P >0.05). Different root regions demonstrated significant differences between the groups. Significant differences between the continuous and intermittent groups were observed on the lingual-middle (0.03 and 0.02 mm 3 , respectively; P <0.05) and the distal-middle (0.15 and 0.09 mm 3 , respectively; P <0.05).
| 2w-C | 2w-I | P | 3w-C | 3w-I | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Total crater volume | 1.01 | 0.69 | 0.75 | 0.44 | 0.069 | 1.13 | 0.46 | 0.76 | 0.32 | 0.007 ∗ |
| Total apical volume | 0.22 | 0.20 | 0.19 | 0.17 | 0.125 | 0.25 | 0.14 | 0.20 | 0.16 | 0.234 |
| Total middle volume | 0.42 | 0.30 | 0.31 | 0.29 | 0.191 | 0.43 | 0.22 | 0.31 | 0.16 | 0.070 |
| Total cervical volume | 0.28 | 0.26 | 0.23 | 0.17 | 0.460 | 0.46 | 0.31 | 0.22 | 0.12 | 0.005 ∗ |
| Total buccal volume | 0.23 | 0.19 | 0.18 | 0.17 | 0.112 | 0.23 | 0.19 | 0.14 | 0.13 | 0.026 † |
| Total lingual volume | 0.12 | 0.12 | 0.13 | 0.26 | 0.156 | 0.10 | 0.09 | 0.08 | 0.06 | 0.605 |
| Total mesial volume | 0.35 | 0.27 | 0.28 | 0.23 | 0.191 | 0.52 | 0.30 | 0.28 | 0.15 | 0.015 † |
| Total distal volume | 0.32 | 0.24 | 0.26 | 0.28 | 0.334 | 0.27 | 0.17 | 0.27 | 0.22 | 0.918 |
| Buccal apical volume | 0.01 | 0.01 | 0.00 | 0.00 | 0.249 | 0.00 | 0.01 | 0.01 | 0.03 | 0.075 |
| Buccal middle volume | 0.08 | 0.11 | 0.07 | 0.10 | 0.433 | 0.06 | 0.07 | 0.05 | 0.08 | 0.079 |
| Buccal cervical volume | 0.15 | 0.12 | 0.11 | 0.14 | 0.100 | 0.17 | 0.15 | 0.09 | 0.07 | 0.034 † |
| Lingual apical volume | 0.08 | 0.10 | 0.06 | 0.09 | 0.363 | 0.07 | 0.07 | 0.06 | 0.05 | 0.650 |
| Lingual middle volume | 0.03 | 0.04 | 0.02 | 0.05 | 0.050 † | 0.03 | 0.04 | 0.01 | 0.02 | 0.433 |
| Lingual cervical volume | 0.00 | 0.00 | 0.00 | 0.00 | 0.317 | 0.00 | 0.00 | 0.00 | 0.01 | 0.593 |
| Mesial apical volume | 0.04 | 0.03 | 0.05 | 0.09 | 0.532 | 0.07 | 0.06 | 0.03 | 0.04 | 0.100 |
| Mesial middle volume | 0.18 | 0.20 | 0.13 | 0.16 | 0.100 | 0.23 | 0.21 | 0.13 | 0.11 | 0.148 |
| Mesial cervical volume | 0.13 | 0.14 | 0.11 | 0.12 | 0.427 | 0.22 | 0.19 | 0.09 | 0.06 | 0.005 ∗ |
| Distal apical volume | 0.10 | 0.11 | 0.08 | 0.09 | 0.532 | 0.11 | 0.10 | 0.10 | 0.12 | 0.756 |
| Distal middle volume | 0.15 | 0.11 | 0.09 | 0.12 | 0.016 † | 0.10 | 0.08 | 0.11 | 0.11 | 0.836 |
| Distal cervical volume | 0.05 | 0.06 | 0.03 | 0.04 | 0.363 | 0.07 | 0.09 | 0.06 | 0.06 | 0.955 |
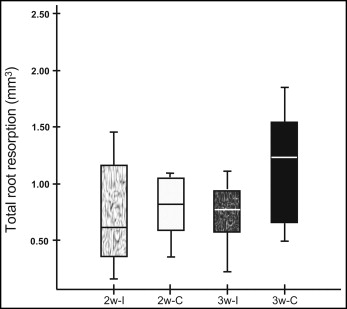
Of the alterations on the root surfaces in groups 3w-C and 3w-I ( Table I ), the mean total root resorption volumes were 1.13 and 0.76 mm 3 , respectively ( Fig 5 ). This difference was significant ( P <0.01). Comparison of continuous and intermittent forces showed significant differences at various levels and surfaces: total cervical (0.46 and 0.22 mm 3 , respectively; P <0.01), total buccal (0.23 and 0.14 mm 3 , respectively; P <0.05), and total mesial (0.52 and 0.28 mm 3 , respectively; P <0.05). There were also significant differences between the continuous and intermittent groups in the buccal-cervical (0.17 and 0.09 mm 3 , respectively; P <0.05) and the mesial-cervical (0.22 and 0.09 mm 3 , respectively; P <0.01) regions.
In the comparison of the continuous force groups ( Table II ), the total cervical volume had a significant difference between 2w-C (0.28 mm 3 ) and 3w-C (0.46 mm 3 ) ( P <0.05) ( Fig 6 ).
| 2w-C | 3w-C | P | 2w-I | 3w-I | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Total crater volume | 1.01 | 0.69 | 1.13 | 0.46 | 0.343 | 0.75 | 0.44 | 0.76 | 0.32 | 0.843 |
| Total apical volume | 0.22 | 0.20 | 0.25 | 0.14 | 0.220 | 0.19 | 0.17 | 0.20 | 0.16 | 0.813 |
| Total middle volume | 0.42 | 0.30 | 0.43 | 0.22 | 0.502 | 0.31 | 0.29 | 0.31 | 0.16 | 0.429 |
| Total cervical volume | 0.28 | 0.26 | 0.46 | 0.31 | 0.036 ∗ | 0.23 | 0.17 | 0.22 | 0.12 | 0.693 |
| Total buccal volume | 0.23 | 0.19 | 0.23 | 0.19 | 1.000 | 0.18 | 0.17 | 0.14 | 0.13 | 0.693 |
| Total lingual volume | 0.12 | 0.12 | 0.10 | 0.09 | 0.635 | 0.13 | 0.26 | 0.08 | 0.06 | 0.343 |
| Total mesial volume | 0.35 | 0.27 | 0.52 | 0.30 | 0.082 | 0.28 | 0.23 | 0.28 | 0.15 | 0.843 |
| Total distal volume | 0.32 | 0.24 | 0.27 | 0.17 | 0.843 | 0.26 | 0.28 | 0.27 | 0.22 | 0.527 |
| Buccal apical volume | 0.01 | 0.01 | 0.00 | 0.01 | 0.535 | 0.00 | 0.00 | 0.01 | 0.03 | 0.572 |
| Buccal middle volume | 0.08 | 0.11 | 0.06 | 0.07 | 0.540 | 0.07 | 0.10 | 0.05 | 0.08 | 0.781 |
| Buccal cervical volume | 0.15 | 0.12 | 0.17 | 0.15 | 0.937 | 0.11 | 0.14 | 0.09 | 0.07 | 0.607 |
| Lingual apical volume | 0.08 | 0.10 | 0.07 | 0.07 | 0.953 | 0.06 | 0.09 | 0.06 | 0.05 | 0.428 |
| Lingual middle volume | 0.03 | 0.04 | 0.03 | 0.04 | 0.555 | 0.02 | 0.05 | 0.01 | 0.02 | 0.356 |
| Lingual cervical volume | 0.00 | 0.00 | 0.00 | 0.00 | 0.333 | 0.00 | 0.00 | 0.00 | 0.01 | 0.538 |
| Mesial apical volume | 0.04 | 0.03 | 0.07 | 0.06 | 0.171 | 0.05 | 0.09 | 0.03 | 0.04 | 0.952 |
| Mesial middle volume | 0.18 | 0.20 | 0.23 | 0.21 | 0.607 | 0.13 | 0.16 | 0.13 | 0.11 | 0.566 |
| Mesial cervical volume | 0.13 | 0.14 | 0.22 | 0.19 | 0.155 | 0.11 | 0.12 | 0.09 | 0.06 | 0.782 |
| Distal apical volume | 0.10 | 0.11 | 0.11 | 0.10 | 0.905 | 0.08 | 0.09 | 0.10 | 0.12 | 0.828 |
| Distal middle volume | 0.15 | 0.11 | 0.10 | 0.08 | 0.213 | 0.09 | 0.12 | 0.11 | 0.11 | 0.501 |
| Distal cervical volume | 0.05 | 0.06 | 0.07 | 0.09 | 0.937 | 0.03 | 0.04 | 0.06 | 0.06 | 0.161 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


