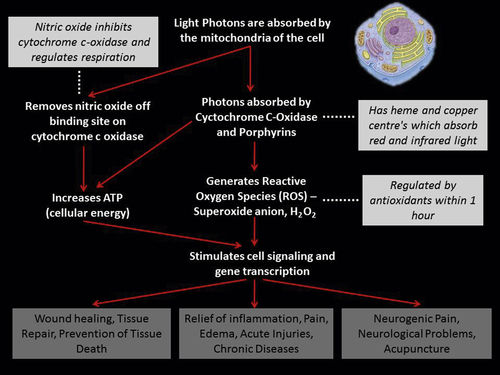
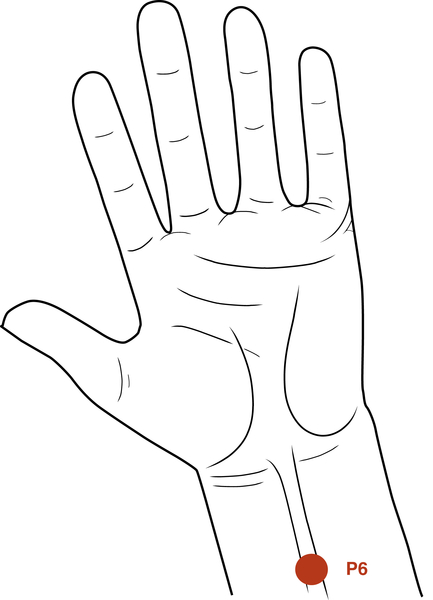
• Figure 15-1 It is often claimed that low-level laser light does not penetrate well through skin. A 650-nm wavelength at only 30 mW shows penetration of low-level laser light through the ventral surface of the hand to the dorsum.
Dosage
The most critical part of PBM is determining the optimal dosage. The tissue dosage is expressed in fluence, or energy density, measured in joules per square centimeter (J/cm2). The total produced energy is determined by multiplying the output power of the laser in milliwatts by the time of exposure in seconds; for example, 50 mW × 40 sec = 2000 millijoules (mJ), or 2.0 J.
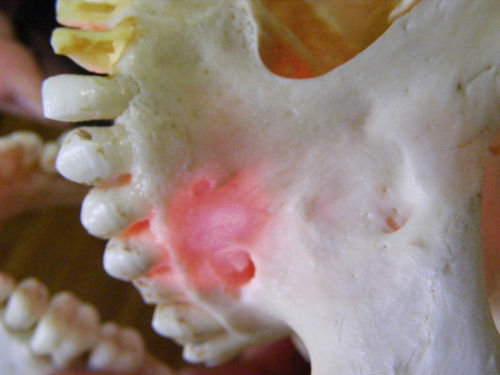
• Figure 15-2 A 650-nm wavelength at only 30 mW shows penetration of low-level laser light through bone.
The next factor to consider is the size of the area being irradiated. With irradiation of an area of 2 cm2, the calculation is 2 J of energy divided by an area of 2 cm2, or 2/2, for a fluence (energy density), or superficial tissue dose, of 1 J/cm2. Suppose that the irradiated area was only 0.5 cm2. An inverse relationship exists between spot size (size of irradiated area) and fluence. Decreasing the size of the irradiated area increases the fluence: 2 J divided by 0.5 cm2 = 4. Thus the dose becomes 4 J/cm2, because the energy was emitted over a smaller area, increasing the local intensity. Because the dose depends greatly on the spot size, a thin light probe will create high doses in J/cm2. This probe/spot size–dose dependency does not necessarily mean that the amount of energy applied to the tissue is high—it merely determines that the intensity of the light energy at the emitting end of the thin probe is high.
With the difference between energy and dose now established, a more complex calculation is in order: determination of the dose at target. If the target is 1 cm below the surface, reflection, scattering, and absorption of the energy could occur before it arrives at the target. It is therefore necessary to consider the depth of the target area and the type of tissue between the light and the target tissue. The main absorbers of these wavelengths are pigmented chromophores, such as the hemoglobin in blood; acccordingly, highly vascular tissue will absorb these wavelengths well, and less vascular tissue will absorb these wavelengths poorly. Mucosa is transparent to these low-level wavelengths; bone also is rather transparent, whereas muscle tissue, with its rich blood supply, is not. Another complicating factor is the amount of another chromophore in the target tissue, melanin. Because melanin is a strong absorber of these wavelengths, more light energy may be superficially absorbed rather than reaching deeper tissue, which may create local heating and even pain.
Therefore the use of J/cm2 to describe the dose in owner’s manuals can be confusing. The J/cm2 (dose, fluence) indicates the intensity at the surface of the tissue but not the dose at the underlying target. A simpler approach is to use the term energy per point, calculating only the number of joules in each point. For clinical use, this is acceptable, but for scientific investigations, unacceptable.6 A “point” often is referred to as the size of the tip of the laser probe (spot size). The relationship between spot size and power density holds true for low-level lasers as well. A small spot size creates a larger concentration of the power per square millimeter or centimeter of tissue irradiated, whereas a wider spot size dilutes the same energy over a larger area.
Stimulation/Inhibition
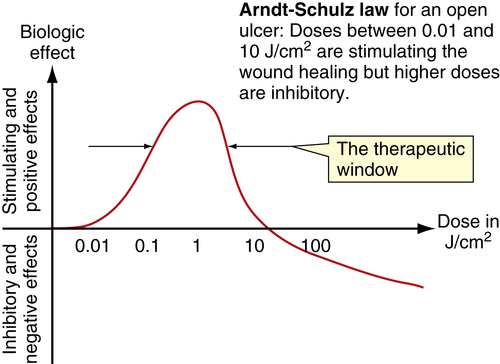
Low-level laser therapy follows the Arndt-Schulz law: Too small a stimulus does not trigger any effect. Increased stimulation augments the effect up to an optimal dose level. Increasing the dose even further means that the stimulation is gradually reduced, and at very high doses, stimulation is inhibited. The quest for the “optimal dose” is still not exact, but much is known about therapeutic windows for this modality. In some patients, the goal is inhibition rather than stimulation, especially for pain management. High doses of laser light will inhibit the pain signals, partly by creating transient varicosities along the neurons, impeding signal transmission.7 Opioid-related mechanisms also have been reported,8 as well as a reduction in the compound action potential9 (Figures 15-4–15-6). Dosages may need to be modified throughout the course of the treatment as the desired outcome changes. Such a flexible approach is indicated during orthodontic treatments, for example. During application of the required force to the tooth, pain reduction is the primary goal, so a bioinhibitory dose is used. At subsequent visits, the aim is to stimulate the osteoblast-osteoclast turn over, so a lower dose is applied.10
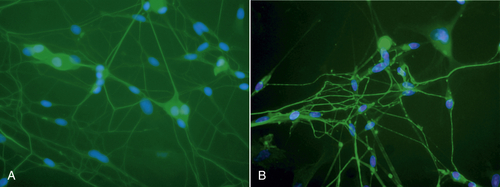
• Figure 15-4 A, Neurons before low-level laser irradiation. B, Neurons after irradiation, using 830-nm wavelength for 120 sec. Note varicosities that formed along axons. These varicosities are transient but are thought to be obstacles in neural transmission. (Courtesy Roberta Chow, MB, Ph.D.)
Acute Versus Chronic Conditions
The general rule is to apply high doses of laser energy for acute conditions that manifest with inflammation and edema, while treating chronic conditions (e.g., wounds, paresthesias, pain) more conservatively. Initially, acute conditions often can be treated until they resolve and the pain is no longer present, whereas chronic conditions should be treated two to three times weekly.
Patients with long-standing chronic pain conditions may experience a flare of pain after PBM, which is commonly known as a treatment reaction. This reaction is transient and actually shows that the patient is responding well to the treatment. The underlying mechanism is believed to involve transformation of the chronic situation into an acute phase, allowing healing to begin. Pain levels are reduced below baseline within 24 h. The patient should be informed about this possibility before treatment, to avoid a reduction in compliance. Other patients undergoing treatment for chronic pain conditions may respond with deep fatigue, interpreted as an accumulated lack of rest, surfacing when the pain subsides. As with pharmaceuticals, management of the reaction and the dosage must be individualized for each patient (Figure 15-7).
As a general rule, acute pain resolves much faster than chronic. In cases of traumatic injury (as from an automobile accident), the sooner laser treatments are initiated, the faster it will resolve.
Pulsing
The importance of pulsing the light is obvious when the laser is applied to cell monolayers in the laboratory.11–13 The pulse repetition rate (PRR) also helps in the clinical setting, although little is known about pulsing in vivo and animal/clinical studies are inconclusive. It is not yet known how to control these mechanisms through use of different PRRs. Also, the biologic effects of a “chopped” continuous-wave laser beam and a superpulsed beam are different. At present, use of a continuous beam is therefore recommended in units that have continuous-wave emission. The 904-nm gallium arsenide (GaAs) laser does not have a continuous-wave mode, so determination of the optimal PRR must rely on anecdotal evidence for selection of pulse parameters.
Moriyama et al.14 demonstrated increased expression of the inducible nitric oxide synthase (iNOS) gene after 905-nm superpulsed laser irradiation. This finding suggests a different mechanism in activating the inflammatory pathway response in superpulsed mode compared with continuous-wave mode.
Number of Sessions
Acute conditions can be resolved with a single PBM session, but many conditions require repeated irradiation for optimal results. Dental treatments often create acute injuries, so repeated irradiation is not necessary. As an example, in an extraction, irradiation of the site at the time of the procedure often is sufficient to reduce pain and inflammation. Other treatments, such as for facial pain or orthodontic discomfort, will require more sessions over a longer period of time. In many cases, follow-up visits requiring irradiation can be delegated to the dental assistant or the dental hygienist. Regulations vary in different areas, so clinicians should confirm local regulations before delegating. In some situations the patient can borrow, rent, or buy a simple low-power laser or LED device (even a traditional 5-mW laser pointer) for a period to optimize the treatment by applying daily doses according to the dentist’s instructions (Figure 15-8).
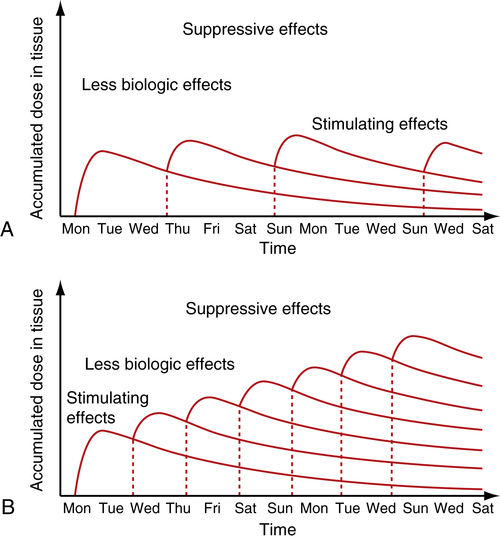
• Figure 15-7 Cumulative effect of dosing in low-level laser therapy (i.e., PBM). A, With several days between doses, the total accumulated dose does not reach an inhibitory range. B, When doses are given too closely together, the accumulated dose reaches an inhibitory range. Such treatment inhibits rather than activates healing, according to the Arndt-Schulz law.
Side Effects and Contraindications
Doses of laser energy near the therapeutic window will not cause negative effects. The worst result with PBM is that nothing happens. There are few absolute contraindications to PBM, but a few caveats follow.
Presence of known malignancies is a contraindication, because PBM stimulates cell growth (this issue is based largely on legal considerations). The literature also discusses pregnancy as a contraindication,15 although dentists work exclusively in the oral and head-neck regions. Also, although sometimes listed as a contraindication, pacemakers are electrical and not influenced by light. Some traditional safety regulations and contraindications seem to have been transferred from electrosurgical and other therapies to surgical lasers.
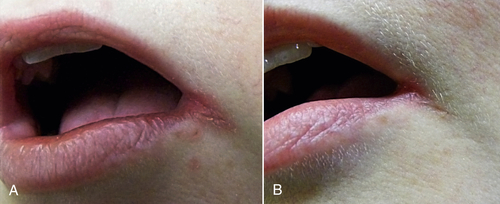
• Figure 15-8 Patient with angular cheilitis treated with one session of PBM in the dental office (30 mW, 3 J, 650-nm wavelength) and then with once-daily self-application at home (1 J per day) for 5 days. A, Preoperative view. B, Postoperative view.
A contraindication especially relevant to dentistry is irradiation over the thyroid gland, located within the dental treatment area. Dentists generally are not informed about possible thyroid conditions; direct irradiation over this area, therefore, should be avoided, because it could stimulate a hyperthyroid condition with a low dose or inhibit a hypothyroid condition with a high dose. However, PBM for thyroid disorders has been studied.16
Documentation
Because of the complex mechanisms involved in PBM, most of the literature is still not at an evidence-based level, except for a few indications. Still, research at more than 100 dental institutions worldwide is represented in the literature, comprising more than 4000 studies. Annually, approximately 250 papers on PBM appear on PubMed, many on dental issues and most reporting positive results. Reports of no effect from PBM often can be attributed to very low doses, miscalculation of the supposed dose, and ineffective treatment applications.17 Nevertheless, some qualified negative studies underline that PBM is not a “hit and run” therapy but depends on knowledge of all relevant parameters. A correct diagnosis and sufficient dosage are key to success.
Laser Safety
Therapeutic lasers with wavelengths of less than 500 mW generally are harmless and classified as “low-risk devices” by the U.S. Food and Drug Administration (FDA).
It is prudent to use protective goggles that are wavelength-specific. Most therapeutic lasers have divergent beams, so from a distance of only a few centimeters, the intensity (and danger) is considerably reduced. PBM has even been successfully used to treat macular degeneration,18 affirming the importance of wavelength and intensity. Some PBM lasers have a collimated lens system, producing a parallel beam. These features have no advantages in dental PBM, and when the laser is used in contact with tissue, the effect of the collimation is lost.
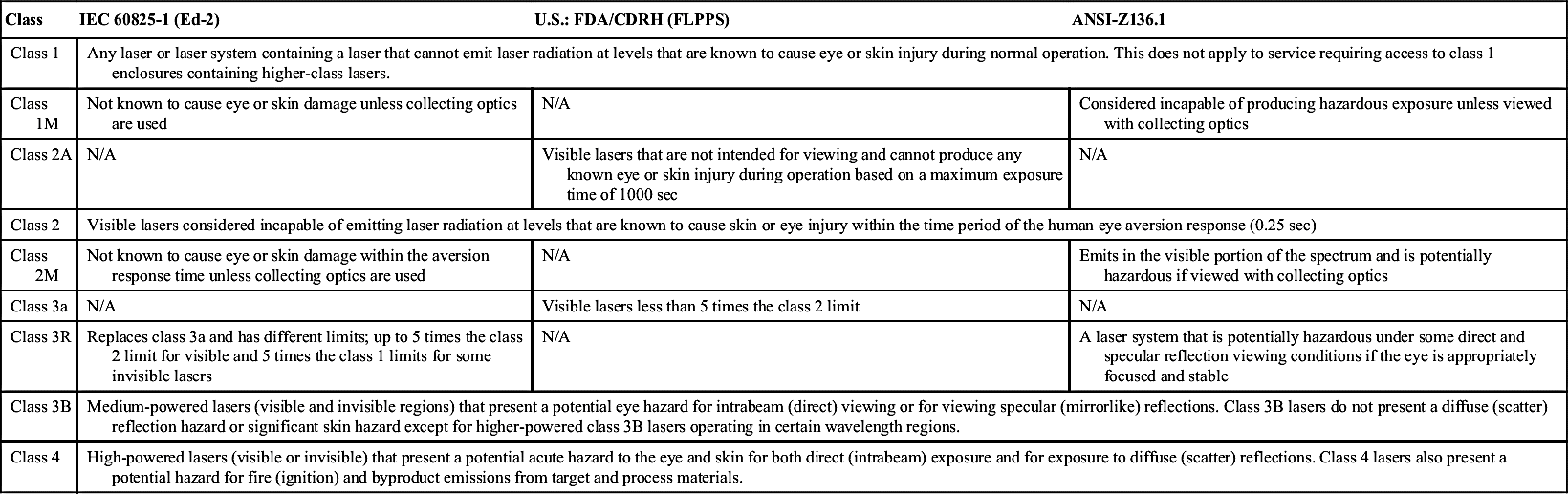
The regulation of laser devices varies from country to country and according to the class. LED devices generally fall in the category of Class 1 devices, for which the standards are much less stringent (Table 15-1).
All clinicians should be familiar with and follow the American National Standards Institute (ANSI) standard, which outlines all safety precautions. Almost all countries follow this standard. Clinicians should consult the appropriate local agencies and authorities to determine the regulations for use of lasers and delegation of laser procedures.
Choosing the “Right” Device
Lasers and LEDs are available with different wavelengths and also different combinations of wavelengths, powers, and probe sizes. The choice of device is therefore not “one size fits all.” Any therapeutic light device can be used for many indications, but some combinations of wavelength and power are optimal for selected indications; clinicians should focus on their special interests. When choosing a device, other practical considerations include probe sterilization, education, technical service, and guarantee period.
Power and Time
During the past decade, stronger lasers have been advocated as more effective, so therapeutic lasers with output of 500 mW or more are now commercially available. For musculoskeletal conditions and pain therapy, high-output lasers may be useful, but for processes such as wound healing and bone regeneration, use of lower power over a longer time is more effective. Availability of power adjustments in a therapeutic laser, as with a surgical laser, is therefore helpful. Applying 5 J in 10 sec with a 500-mW laser is quite different from applying the same 5 J with a 50-mW laser in 100 sec. The 500-mW laser would work better for pain but might be less effective for treatment involving tissue regeneration.19 This divergence of effect reemphasizes the importance of adequate and appropriate training in the decision-making process.
Hygiene
Some therapeutic lasers have a removable, sterilizable probe, similar to that of the dental curing light; if it will not affect the optics, the probe should go through a heat sterilizer and the handle should be covered with a disposable barrier. If the tip cannot be sterilized, the whole unit should be covered in a disposable barrier. Sterilizing requirements vary, so familiarity with and understanding of local regulations are imperative.
Biostimulation
Although dental lasers such as the Nd:YAG, CO2, and erbium family are considered “hard” or “surgical” lasers, various studies indicate that they also can produce a degree of biostimulation in the areas peripheral to the focal spot, where the energy is reduced to stimulatory levels (Figure 15-9).
Some of the positive effects observed with the hard lasers may be explained by biostimulation. Pourzarandian et al.20,21 reported stimulation of human gingival fibroblasts by low-level Er:YAG irradiation, as well as increased prostaglandin E2 (PGE2) production through induction of cyclooxygenase-2 (COX-2) messenger ribonucleic acid (mRNA) in human gingival fibroblasts. Additionally, Er:YAG lasers may be used at lowest output and scanned at a slight distance (out of focus) to produce biostimulation. The problem with using these hard lasers in this manner is that no microprocessor informs the practitioner how to control the dosage, and the fibers are not specifically adapted for biostimulation.
Biostimulation is not limited to the traditional near-infrared wavelength window; such effects are even reported with use of defocused CO2 lasers.22–24 The CO2 laser wavelength has extremely poor penetration through tissue because it is so well absorbed at the surface, and the biologic effects reported on deeper tissues may first appear improbable. However, the coherent light is absorbed in peripheral microvessels, and the clinical effect observed shows that PBM has primary effects at the target as well as systemic effects through blood and lymph circulation. Thus, with minimal calculations in clinical practice, the owner of a “hard” laser can have a “soft” laser for free. Least complicated is the surgical diode laser, with simpler calculations and wavelengths within the traditional range of biostimulation.
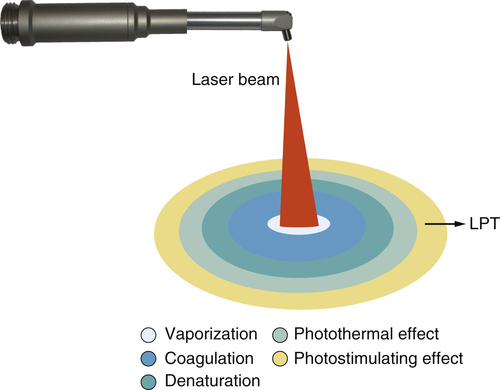
• Figure 15-9 Various zones of effect surrounding the focal spot when using a surgical laser (CO2, Nd:YAG, erbium, diode). At the focal spot, vaporization occurs. Concentric to that is a zone of coagulation, where the proteins in the tissue have absorbed energy and have coagulated but have not been vaporized. Concentric to that is the zone of denaturation, where tissue proteins have absorbed a sufficient amount of energy to be heated to the point of denaturation but have not absorbed enough energy to be coagulated. Concentric to that is the zone of photothermal effect, where the tissue has absorbed enough energy to be heated but is not otherwise affected. Concentric to that is the zone of photostimulation, where some low-level laser activity can occur.
Photoactivated Disinfection
There is a growing interest in the combination of different dyes with therapeutic lasers. Neither the dyes nor the therapeutic lasers used alone will have an effect on bacteria, but in combination, singlet oxygen will be produced, which has a strong bactericidal effect.
The photoactivated disinfection (PAD) method is already commercialized and recommended for use in periodontal pockets, periimplantitis, deep carious lesions, and infected root canals.24–28 The laser must work within the absorption range of the dye used, and wavelengths generally are in the red spectrum, with a power output of 50 to 100 mW. The selected dye is applied and allowed to diffuse for a few minutes, and then laser irradiation is performed. Some transient discoloration may be seen in dentinal and mucosal areas.
Curing Light
All dentists have in their armamentarium a composite curing light, typically with a wavelength peak of approximately 470 nm. Energies delivered are within the low-level laser therapeutic window, but studies of the photobiologic effect on the surrounding tissues are largely lacking.
In a 2009 in vitro study, Enwemeka et al.29 demonstrated that light-emitting diode (LED) energy with a 470-nm peak successfully kills methicillin-resistant Staphylococcus aureus (MRSA). Irradiation produced a statistically significant, dose-dependent reduction in both the number and the aggregate area of colonies formed by each strain of cells. The higher the dose, the more bacteria were killed, but the effect was not linear, being more impressive at lower than at higher doses. Almost 30% of both strains was killed with as little as 3 J/cm2. As much as 90.4% of the two different colonies was killed with an energy density of 55 J/cm2. This study requires further in vivo investigation but suggests an innovative use of an available dental light source to treat MRSA infection, which carries high morbidity and mortality rates. A short wavelength such as a curing light has a very shallow penetration depth, so it will be more effective on surface lesions
Lasers Versus Light-Emitting Diodes
Laser light is coherent and is delivered as a collimated beam, whereas light from an LED light is noncoherent, without collimation. The subject of whether one is more efficacious than the other is a topic of controversy, often resulting in very heated discussion. Many research studies have investigated the effect on tissue with LEDs and lasers, with differing outcomes. A study by Nishioka et al. in 2012, however, investigated the effect of laser and LED irradiation using the same total energy deposited in tissue and found that both resulted in a significant decrease in the necrosis of the skin flap, which indicated that the light source consistency was not essential for successful results.30
Acupuncture
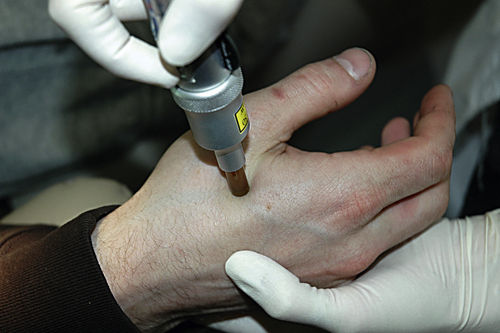
Few dentists use acupuncture, but several acupuncture points can be used in low-level laser applications by all dentists. Therapeutic lasers produce effects similar to those of acupuncture needles and are safer to use. For example, P6 (i.e., Neiguan) on the wrist is an excellent point for use in reducing nausea and vomiting.31 The location of this point is 2.5 cm up from the wrist and 0.5 to 1.0 cm deep. The point is between two tendons. Delivery of 3 to 4 J to P6 often allows a more relaxed environment for taking impressions or working in the molar area, especially in patients with a heightened gag reflex. Another easily accessible point is the Li4 (i.e., Hegu), a pain-reducing point located in the middle of the second metacarpal bone on the radial side (Figures 15-10 and 15-11).
Functional magnetic resonance imaging (fMRI) has confirmed the similarity of needles and lasers in their effect with application on the same acupuncture point.32 However, understanding this phenomenon becomes more difficult because a qi effect, the purported underlying mechanism in acupuncture, is absent with the laser. The gate control theory would not explain the effects because pain stimulus in the acupuncture point does not occur with use of a laser.
Dental Indications
Because PBM can influence so many pathologic conditions, the use of the therapeutic laser is not limited to the following indications. More than 30 conditions have been described in the dental literature; the most important are briefly described here. As more research is conducted, the number of indications for use based on peer-reviewed literature will undoubtedly increase.
Anesthesia
Applying PBM to the mucosa, but not the hard palate, before an injection results in a slight anesthetic effect.33 PBM applied before the injection also will improve healing, should the needle cause trauma to a vessel or nerve. PBM improves local microcirculation,34 so the effect of the numbness can be shortened if PBM is applied to the site after completion of the dental procedure. Energy of 4 to 6 J is needed in both cases.
Aphthous Ulcers
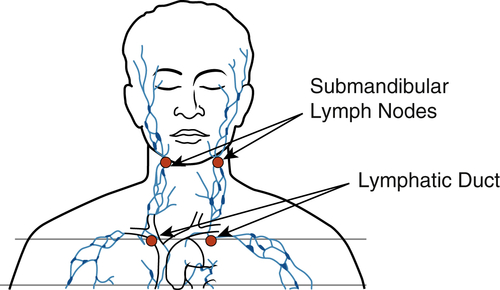
The healing time of aphthous ulcers can be shortened and the immediate pain reduced by administering 4 to 6 J over the lesion and 4 J to the submandibular lymph nodes on the affected side35,36 (Figure 15-12). Patients prone to development of aphthous ulcers should avoid toothpastes containing sodium lauryl sulfate, which may trigger these lesions in predisposed persons.
Edema
The lymphatic system plays an important role in the inflammatory process, and PBM applied over the involved lymph nodes decreases edema. Irradiation should start over the most distal nodes of the chain involved and proceed toward the focus of the swelling, using 4 J per node. The permeability of the lymph vessels will be reduced and lumen size increased, and repeated irradiation will stimulate growth of collaterals37–41 (Figure 15-13). The lymphatic system also brings the lymphocytes and natural killer cells to fight infection. Meneguzzo et al.41 reported that irradiation with an 810-nm laser reduced rat paw edema whether it was performed in the paw itself or over the inguinal lymph nodes.
Endodontics
PBM does not have a bactericidal effect, and surgical lasers are the preferred instruments to reduce bacteria in infected root canals. Few manufacturers of therapeutic lasers offer probes that could reach the root canals. However, infrared light can reach all apices, and visible red light can reach the more superficial apices through the mucosa, to produce an antiinflammatory and pain-reducing effect.
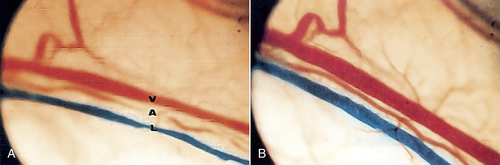
• Figure 15-13 A, Vein, artery, and lymphatic vessel before irradiation. Note diameter of the vessels. B, After low-level irradiation of vessels using a laser with 904-nm wavelength. Note dilation of vessels, which leads to increased circulation in the region they supply. (Courtesy Pierre Lievens.)
Sousa et al.42 analyzed the effect of PBM on the secretory activity of macrophages activated by interferon gamma (IFN-γ) and lipopolysaccharide (LPS) and stimulated by substances leached from an epoxy resin–based sealer (AH-Plus) and a calcium hydroxide sealer (Sealapex). The production of tumor necrosis factor alpha (TNF-α) was significantly decreased by PBM, regardless of experimental group. The level of secretion of matrix metalloproteinase-1 (MMP-1) was similar in all groups.
Laser application after overinstrumentation and overfilling is a good example of an indication for PBM in endodontics. Because PBM can stimulate bone formation, apical bone probably can heal faster after completed endodontic treatment if PBM is applied. Energy needed is related to the depth of the apex, ranging from 4 to 8 J per apex. For the same reason, intraoperative and postoperative PBM in apical surgery has potential,43,44 but solid scientific documentation is still lacking. However, irradiation over the suture line will stimulate fibroblast proliferation and increase tensile strength.45
In patients with acute pulpitis, when the affected tooth or root is difficult to pinpoint, the laser can be applied over the apices in the involved area. The affected tooth may react with an increase in pain, probably because of increased microcirculatory pressure in the pulpal chamber and increased lymphatic flow. PBM also can be used as an adjunct therapy in pulp capping46,47 and pulpotomy48,49 (see Chapter 13). In both cases, the exposed pulp is irradiated at low intensities, applying 4 J after the exposed pulp has been cauterized but before any liners, cements, or other medicaments have been placed. The irradiation will reduce inflammation, preserve odontoblastic integrity, and stimulate cellular proliferation.
Extractions
Nontraumatic approaches and good postoperative procedures are the key to satisfactory healing after extractions. However, the occasional complication is unavoidable. Adding PBM after the extraction will reduce the inflammatory phase, induce pain reduction, stimulate the fibroblasts in the wound periphery, and stimulate the osteoblasts in the socket.50–55 High doses of laser energy applied directly into the socket will reduce postoperative pain and inflammation (Figures 15-14 and 15-15).
The major goal of PBM after extractions is first to reduce pain and inflammation and then to stimulate the fibroblasts to seal the socket. In cases of post-extraction failure (dry socket), the traditional methods are used in combination with very high doses of PBM to reduce patient discomfort. The light energy is applied until the patient notices a decrease in the level of pain, and then a dressing is placed. When the dressing is changed during subsequent appointments, lower doses are given to stimulate fibroblast growth so that the epithelium can cover the exposed bone.
Herpes Simplex Virus
Patients with a herpes simplex virus type 1 (HSV-1) eruption may be reluctant to visit the dentist. However, PBM is the most efficient method of treating this infection.36,56–58 In particular, if it is treated during the initial prodromal stage (when the patient feels the initial tingling), healing will take only a few days, or the symptoms may even disappear within hours. Of importance, in patients with recurrent HSV-1 attacks, longer intervals between the outbreaks are typical after PBM treatment.59,60
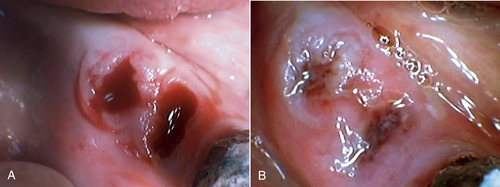
• Figure 15-15 A, Socket immediately after tooth extraction. B, Extraction socket after laser irradiation using 650-nm wavelength at 30 mW, 8 J, 1 day after extraction. (Courtesy Talat Qadri)
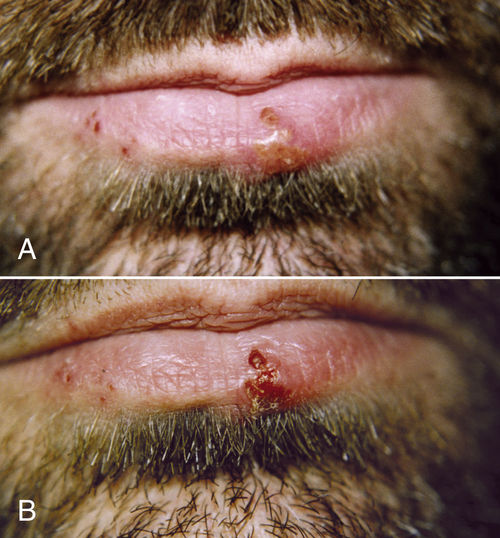
• Figure 15-16 Herpes outbreak on the lower lip of a male patient. A, Preoperative view of. Blisters are in the vesicle stage. Outbreak started the day before the patient presented to the office. Area was treated with 808-nm laser at 500 mW, 4 J. B, One-day postoperative view. Lesions have crusted over, and pain has been reduced.
In the acute stage of HSV-1 infection, repeated treatment is necessary; irradiation during the prodromal stage may only be a single session of 2 to 6 J over each blister and 4 J to the submandibular lymph nodes on the affected side, in accordance with size and duration. The actual effect is not known, but research has confirmed that laser light enables the cell to resist viral attack for a longer period, presumably providing time for the immune system to react61 (Figure 15-16).
Implants
A single high dose of irradiation after implant placement will reduce postoperative pain, inflammation, and edema. Repeated sessions at a lower dose will stimulate osseointegration by stimulating the osteoblasts. As with all healing processes, repeated irradiation is necessary; 4 to 6 J over each implant is recommended, with the laser tip in slight contact.62–66 PBM also is a useful additional therapy in the control of periimplantitis. The effect is most prominent if PBM is applied immediately after surgery and then two or three times per week for 2 weeks.
Khadra67 summarized five studies using the 830-nm wavelength as follows:
PBM can promote bone healing and bone mineralization and thus may be clinically beneficial in promoting bone formation in skeletal defects. It may be also used as additional treatment for accelerating implant healing in bone. PBM can modulate the primary steps in cellular attachment and growth on titanium surfaces. Multiple doses of PBM can improve PBM efficacy, accelerate the initial attachment and alter the behavior of human gingival fibroblasts cultured on titanium surfaces. The use of PBM at the range of doses between 1.5 and 3 J/cm2 may modulate the activity of cells interacting with an implant, thereby enhancing tissue healing and ultimate implant success.
Lopes et al.64 suggested that the 830-nm wavelength can allow earlier loading of titanium implants. Kim et al.65 found that PBM influenced the expression of osteoprotegerin (OPG), receptor activator of nuclear factor kappa B (RANK), and RANK ligand (RANKL), increasing metabolic bone activity. RANKL is a surface-bound molecule that activates osteoclast cells involved in bone resorption. Overproduction of RANKL is implicated in a variety of degenerative bone diseases, including rheumatoid arthritis and psoriatic arthritis.
Guzzardella et al.66,68 reported similar stimulatory effects at the bone-implant surface with hydroxyapatite-coated implants using a 780-nm laser. Whether the positive effects on bone-implant integration represent a general effect or specific cell stimulation is not yet known, because several wavelengths and dosages have been used (Figure 15-17).
Inflammation
PBM will shorten the course of the inflammatory process, and it is important to recognize that acute pain reduction requires high doses, whereas reduction of the inflammatory period requires less dosage. Reducing the pain may satisfy the patient but may prolong the inflammatory process. The infrared laser dose for inflammation reduction is 8 to 12 J/cm2.
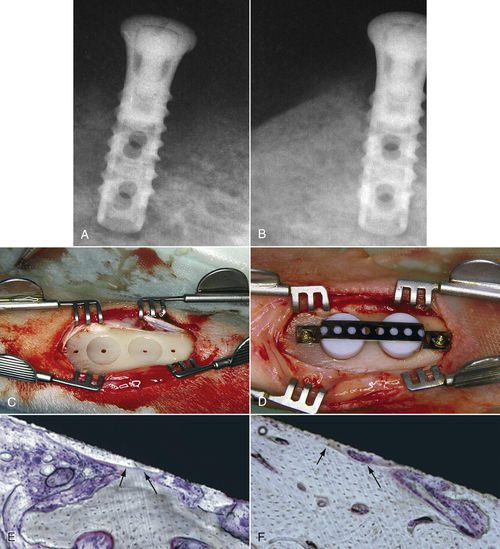
• Figure 15-17 Low-level laser stimulation of bone healing. A and B, A failing 2-month-old implant. On the preoperative radiograph (A), note the radiolucent quality of bone surrounding the implant. On a postoperative radiograph (B) obtained 4 weeks after application of 4 J of energy twice weekly, using 30 mW with 830-nm wavelength, bone quality is greatly improved. C to F, For a study in 12 adult (8-month-old) New Zealand white rabbits, tibial bone was used as the experimental area. As seen in C and D, two coin-shaped-titanium implants were inserted into the cortical bone, covered with a polytetrafluoroethylene cap, stabilized with a titanium plate, and retained by two titanium screws. In E and F, histologic views of the implant in situ show ground section of periimplant bone with osseointegration at 8 weeks after implantation in the control group (E) and the irradiated group (F). Histomorphometric analysis showed more bone-to-implant contact in the irradiated group than in the control animals (with approximately 10% improvement). (Courtesy Maawan Khadra.)
As mentioned previously, irradiation of the lymphatic system is a major aspect of PBM for antiinflammatory interventions. Lim et al.69 concluded that 635-nm irradiation and existing COX inhibitors inhibit expression of COX and PGE2
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses