Chronic kidney disease (CKD) arises from abnormalities in the structure and/or function of the kidneys, present for more than 3 months, and leading to compromised renal function. The main causes of CKD are diabetes and hypertension, which are implicated in two-thirds of all cases1,2. The remaining cases either are attributed to immunological and inherited conditions, or remain of unknown aetiology. Hypertension is thought to lead to CKD by a progressive narrowing of the afferent arterioles, leading to a decreased blood flow to the glomeruli and therefore a decreased renal filtration rate3. The decreased blood flow to the nephrons triggers the release of renin, leading to the activation of the renin-angiotensin system (RAS). Activation of RAS leads to increased heart rate and vasoconstriction, which creates a positive feedback loop leading to more hypertension, in a misguided attempt to increase the blood flow to the kidneys. This leads to glomerulosclerosis and loss of the functional unit of the kidney, the nephron1,4. The mechanism of renal damage in diabetes is somewhat similar in that hypertension and diabetes share common risk factors. In addition, the hyperglycaemic state in diabetes leads to overproduction of reactive oxygen species (ROS). Excess generation of ROS leads to overproduction of growth factors, pro-inflammatory cytokines and increased oxidative stress (see Chapter 1 ‘Periodontitis, obesity and diabetes mellitus’). These factors combined lead to the features seen in diabetic nephropathy, including mesangial expansion and cell proliferation, podocytopathy, hypertrophy and atrophy, glomerular basement membrane thickening and sclerosis (akin to hypertension)5. The loss of nephrons, caused by hypertension or diabetes, leads to an increasing demand for filtration by the remaining functional nephrons. This is termed glomerular hyperfiltration. The nephrons tolerate compensation in this way for a period of time, but sustained hyperfiltration leads to glomerular sclerosis of the functional nephrons, leading to their loss. This accounts for some of the progressive nature of CKD (Fig 3-1).
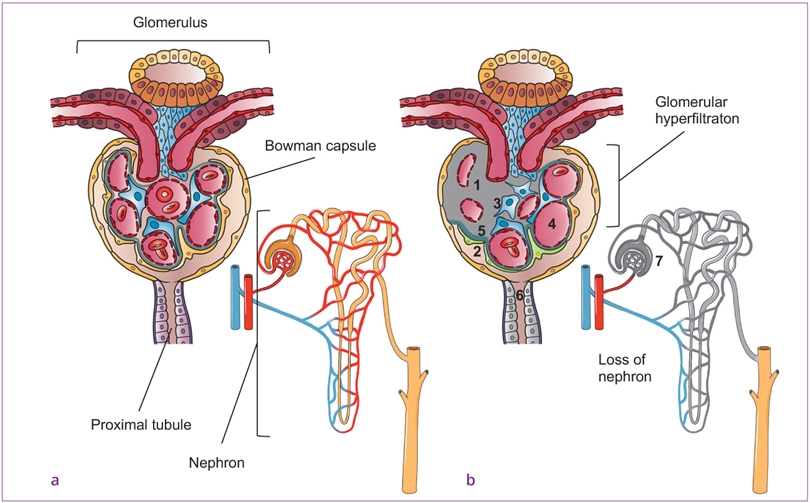
Fig 3-1a and b a Healthy glomerulus and b pathological changes during chronic kidney disease. (1) Glomerular sclerosis and damaged endothelium, (2) podocytopathy, (3) mesangial expansion and cell proliferation, (4) compensatory enlargement of arteriole, (5) glomerular basement membrane thickening, (6) apoptosis and/or necrosis of tubular epithelial cells, (7) tubular atrophy. Image courtesy of Dr Josefine Hirschfeld, Birmingham Dental School and Hospital, Birmingham, UK.
Globally, CKD affects approximately 13% of the world population6. As the prevalence of the risk factors for CKD, such as age7, diabetes and hypertension8, increase globally9 it is feared that the prevalence of CKD will increase in the future. CKD is classified into stages 1 to 5 based on estimated renal function, with patients in stage 5 or end-stage renal disease (ESRD) having the poorest kidney function and commonly requiring renal replacement therapy (RRT) in the form of either dialysis or renal transplant. The need for dialysis or renal transplant significantly increases the morbidity associated with CKD. The management of patients requiring RRT also incurs significant financial cost to the health care system or to patients. In 2009 to 2010, the annual cost for treatment of patients with stages 3 to 5 CKD in England alone was estimated at approximately £1.45 billion (approximately 1.3% of the overall National Health Service [NHS] budget in that period) and more than half of this cost was spent on patients requiring RRT10. Even though ESRD comes with a great burden in terms of morbidity, mortality and finance, most patients with CKD do not progress to ESRD. Instead, they suffer from early mortality, mainly as a result of adverse cardiovascular events. The increased cardiovascular risk in CKD arises partly from the shared risk factors of diabetes and hypertension, but the risk in patients with CKD appears to be higher than in those with similar risk factors, but without CKD. As described above, hypertension and CKD have a bidirectional relationship, which, in part, explains the burden of cardiovascular disease (CVD) seen in patients with CKD. In addition, left ventricular hypertrophy, leading to reduced cardiac output is also highly prevalent in patients with CKD. This may result from the hypertension, renal anaemia or the increased vascular stiffness seen in patients with CKD. Renal anaemia is thought to arise from reduced erythropoietin production from damaged peritubular kidney cells, and increased vascular stiffness is thought to arise from reduced expression of endothelial nitric oxide synthase, seen in early stages of CKD. Atherogenic lipid profiles, seen in patients with CKD, with defective high-density lipoprotein (HDL) cholesterol function and excess oxidation of low-density lipoprotein (LDL) cholesterol, further increase CVD risk (see Chapter 2 ‘Periodontitis and atherosclerotic cardiovascular disease’). Finally, the increased inflammatory/oxidative stress burden in patients with CKD, caused by a lack of renal clearance of post-synthetically modified protein and toxins, may also contribute to increased CKD risk11.
The gold-standard method of estimating renal function is based on the renal clearance of inulin, a plant-based polysaccharide. Inulin is continuously infused and multiple, strictly timed, measurements of blood and urine are collected. As inulin is neither absorbed nor secreted by the renal tubules, the rate of excretion in the urine is used to quantify the glomerular filtration rate (GFR), with a low rate of filtration, usually less than 60 ml/min/1.73m2 indicating poorer renal function. As this process is very time-consuming and labour intensive, other estimations of glomerular filtration rate (estimated GFR [eGFR]) are commonly used. These depend on surrogate markers of renal function, such as serum creatinine levels, and are adjusted for the patient’s age, gender and ethnicity, all of which also impact on levels of serum creatinine. A number of mathematical formulas have been used to estimate GFR and, currently, the commonly used ones derive from the Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) group.
As an example, the 1999 MDRD equation12 for calculating eGFR is:
eGFR = 175 × serum creatinine−1.154 × age−0.203 × 1.212 (if Afro-Caribbean) × 0.742 (if female), where serum creatinine is measured in mg/dl and age is measured in years. Serum creatinine levels included in the formula are therefore key in determining eGFR, and as serum creatinine levels themselves are linked to age, gender and ethnicity differences, the equation above accounts for these inputs.
Aside from eGFR, another method of quantifying renal function derives from the amount of protein that leaks into urine, also called ‘proteinuria’, due to damage to the renal tubules. This is expressed as urinary albumin–creatinine ratio (uACR). The greater the amount of protein leaking into urine, the more damaged the kidneys are thought to be. Together, eGFR and uACR can be used to categorise patients into stages of CKD (Table 3-1).
Table 3-1 Prognosis of chronic kidney disease (CKD) by estimated glomerular filtration rate (eGFR) and albuminuria category13
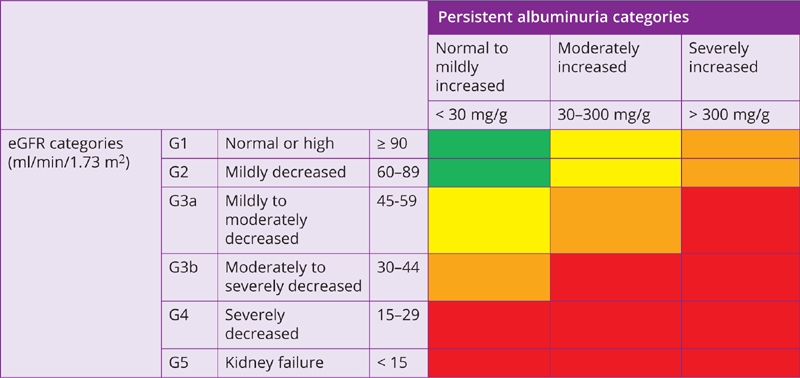
Green = low risk of adverse clinical outcomes (if no other markers of kidney disease, no CKD); Yellow = moderately increased risk; Orange = high risk; Red = very high risk.
Current management of patients with CKD revolves around management of ‘traditional’ risk factors such as diabetes, hypertension and smoking, which are implicated in the initiation and progression of CKD. Therefore, patients with CKD have their hypertensive and glycaemic control closely monitored and are counselled on the advantages of smoking cessation. These measures are aimed at halting or slowing the progression of disease, beyond which there is very little by way of treating the condition. However, at least 50% of the increased mortality seen in patients with CKD is not associated with the traditional risk factors of diabetes, hypertension or smoking14. The focus of recent research in CKD has been the identification of non-traditional risk factors that might explain why some patients with CKD progress faster or decease earlier than others. Indeed, the individual systemic inflammatory and oxidative stress burden is thought to be one such risk factor. This is because cardiovascular mortality in patients with CKD is not only related to the severity of kidney disease15, but also to an increased systemic inflammatory and oxidative stress burden. Hence, biomarkers of systemic inflammation such as C-reactive protein (CRP) are reliable indicators of cardiovascular and all-cause mortality in patients with CKD16. These are discussed in Section 3.3 ‘Cellular and molecular mechanisms’, in the context of periodontitis. In patients with CKD, cardiovascular risk factors like hypercholesterolaemia are inversely linked to mortality, a phenomenon termed ‘reverse epidemiology’17. This suggests that alternative pathways involving inflammation might be determinants for the progression of atherosclerotic disease (see Chapter 2 ‘Periodontitis and atherosclerotic cardiovascular disease’) in such patients. Periodontitis may influence the systemic inflammatory/oxidative load via:
● spread of intact bacteria via the bloodstream to sites distant to the oral cavity, where inoculation sequelae arise
● spread of pro-inflammatory bacterial products, which activate a systemic inflammatory/oxidative stress response
● spread of local inflammatory mediators and oxidative stress products (e.g. oxidised-LDL) from the gingivae, via the bloodstream, to sites distant from the oral cavity17a,17b.
The clinical evidence behind the association between periodontitis and CKD derives from a mixture of epidemiological/observational studies and interventional/clinical trials as summarised in recent systematic reviews. The evidence from patients with CKD is usually presented in RRT (dialysis or transplant) or non-RRT populations. Where possible, for the purposes of this chapter, the findings will be presented based on systematic reviews and/or meta-analyses and subdivided by the population studied (RRT/not RRT) and the category of trial design (observational or interventional).
3.2.1 Observational studies investigating the association between periodontitis and mortality in patients with CKD
In 2017, Zhang at el18 conducted a meta-analysis of cohort studies (studies where groups of individuals are typically followed over a period of time) assessing the association between periodontitis and mortality in patients with CKD. Eight studies were included in the final analysis. All of these investigated the association between periodontitis and all-cause mortality, and four also investigated the association between periodontitis and cardiovascular mortality in patients with CKD. Meta-analysis revealed that periodontitis was associated with a small but statistically significant increased risk of all-cause death (risk ratio [RR] 1.25, 95% CI 1.05 to 1.50, P = 0.005). In other words, patients with CKD and periodontitis were 25% more likely to die when compared with patients with CKD without periodontitis. The researchers also showed an increased risk of cardiovascular mortality in patients with periodontitis, compared to those without, but this failed to reach statistical significance (RR 1.30, 95% CI 0.82 to 2.06, P = 0.259). As with any piece of research, the authors acknowledge some of the limitations of their work. The main limitation of this meta-analysis is that the studies included differed in what they classified as ‘periodontitis’, what they classified as ‘CKD’, and the nature of the analyses conducted to evaluate the associations between periodontitis and mortality in patients with CKD. These inter-study differences can be grouped under the umbrella term of ‘methodological heterogeneity’, which is a term that particularly plagues all research into periodontal and systemic disease links. The other shortcoming of this piece of work is that the original articles measured periodontitis at one time-point and then investigated associations with mortality. This assumes that the periodontal status does not change over time. This is unlikely to be the case as periodontitis is not static but a dynamic process in which the disease may progress or may be treated.
Data not in accordance with the above meta-analysis were provided by Ruospo et al19 in 2017. The authors performed a well-designed multinational propensity-matched cohort study to assess the associations between periodontitis and early (all-cause and cardiovascular-related) mortality among adults treated with haemodialysis. They included 3338 dentate adults with ESRD treated in a haemodialysis network in Europe and South America. Confounders adjusted for were sex, age, number of teeth, income, smoking, physical activity, body mass index, myocardial infarction, diabetes, mean arterial pressure, time treated with dialysis, and serum phosphorus. Furthermore, analyses were repeated omitting participants with fewer than 12 natural teeth, and the potential relevance of the competing risk of non-cardiovascular-related death was taken into account. The authors reported that periodontitis was not associated with an increased risk of early death in their study19.
In 2016, Sharma et al20 conducted an analysis of the US National Health and Nutrition Examination Survey III (NHANES III). A total of 13,784 subjects were included in the analysis, of which approximately 6% had CKD. Linked mortality data was used to follow up subjects, with a median follow-up time of 14.3 years. After adjusting for confounders, such as age, gender, ethnicity, smoking status and socio-economic status, individuals with CKD and periodontitis had a 10-year all-cause mortality of 41% (95% CI 36% to 47%) compared with individuals with CKD who were periodontally healthy, who had a 10-year all-cause mortality of 32% (95% CI 29% to 35%). This increase in mortality seen in patients with CKD and periodontitis was of a similar magnitude to individuals who had diabetes instead of periodontitis (Table 3-2 and Fig 3-2).

Fig 3-2 Influence of comorbidities on survival in participants with stage 3 to 5 odontitis (CKD).
Table 3-2 Ten-year all-cause and cardiovascular mortality (%) of individuals with chronic kidney disease (CKD) by risk factors
| Risk factor/s | 10-year all-cause mortality (95% CI) | 10-year cardiovascular mortality (95% CI) |
|---|---|---|
| CKD | 32% (29–35%) | 16% (14–19%) |
| CKD + periodontitis | 41% (36–47%) | 22% (19–27%) |
| CKD + diabetes | 43% (38–49%) | 24% (19–30%) |
This analysis also suffers from the limitations of the studies included in the previously described meta-analysis, in that the periodontal assessment was only carried out at one time-point with no means of accounting for the changes in periodontal health until the end of the study. In addition, this study was conducted using data from the NHANES III survey that, being a large-scale cross-sectional survey, used a partial-mouth probing protocol to evaluate periodontal health. This means that some patients are likely to have been wrongly classified as ‘healthy’ when in fact they had periodontitis, as the whole mouth was not assessed. Therefore, the magnitude of associations reported by the authors is likely to be an underestimate of the true magnitude of association. Lastly, this large survey referred to non-institutionalised US civilians, who do not represent the worldwide population, hence affect the generalisability of the results (i.e. the conclusions are not applicable to every patient suffering from CKD and periodontitis).
3.2.2 Cross-sectional studies investigating the association between periodontitis and CKD
Cross-sectional studies (one-time survey of a given population/sample) provide the bulk of the evidence in the association between periodontitis and CKD (Table 3-3). The majority of such studies are carried out in populations undergoing RRT and fewer are undertaken in pre-RRT populations. This discrepancy is also temporal, with the first such articles in RRT populations27 preceding articles in non-RRT populations28 by over a decade.
Table 3-3 Selected clinical studies published studying the association between pre-dialysis chronic kidney disease (CKD) and periodontitis
| Study | Study population | Outcomes | Study design |
|---|---|---|---|
| Kshirsagar et al21 | 5537 middle-aged black and white men and women from D-ARIC | Compared with healthy/gingivitis, initial and severe periodontal disease were associated with a GFR < 60 ml/min/1.73 m2 with OR 2.00 (95% CI 1.23–3.24) for initial periodontal disease and OR 2.14 (95%CI 1.19–3.85) for severe disease, after adjustment for important risk factors for CVD and CKD | Cross- sectional |
| Fisher et al22 | 12,947 adults 18 y or older from NHANES III | Adults with periodontal disease and edentulous adults were twice as likely to have CKD (OR 1.60, 95% CI 1.16–2.21; OR 1.85, 95% CI 1.34–2.56, respectively) after simultaneously adjusting for other traditional and non-traditional risk factors | Cross- sectional |
| Garcez et al23 | 80 adults with GFR 60–89 ml/min/1.73 m2 and 80 age- and gender-matched controls with GFR ≥ 90 ml/min/1.73 m2 | No significant differences between patients’ and controls’ supragingival plaque accumulation, calculus deposits, gingival inflammation, depth of periodontal pockets, clinical attachment loss or dental mobility. The results suggest that in patients with mildly decreased GFR, there are no alterations of the oral health status. | Case-control |
| Grubbs et al24 | 6199 dentate adult participants (aged 21 to 75 y) from 2001 to 2004 NHANES | Periodontal disease was associated with a higher risk of CKD (OR 1.51, 95% CI 1.13–2.02) | Cross- sectional |
| Ioannidou and Swede25 | 12,081 adults from NHANES III | Mexican-Americans and non-Hispanic Blacks with CKD had increased odds of moderate periodontitis (OR 1.59, 95% CI 1.14–2.13; and OR 1.24, 95% CI 0.95–1.62 , respectively) compared with those without CKD | Cross- sectional |
| Sharma et al26 | 469 patients, with stage 3–5 (pre-dialysis CKD) compared with 876 non-CKD, community controls | Among dentate subjects, patients within RIISC were significantly more likely to have any (OR 4.0, 95% CI 2.7–5.9) or severe (OR 3.8, 95% CI 2.5–5.6) periodontitis compared to the ADHS sample | Case-control |
ADHS = adult dental health survey; CI = confidence interval; CKD = chronic kidney disease; CVD = cardiovascular disease; D-ARIC = Dental Atherosclerosis Risk in Communities study; GFR = glomerular filtration rate; NHANES = National Health and Nutrition Examination Survey; OR = odds ratio; RIISC = Renal Impairment in Secondary Care study.
In 2013, Chambrone et al29, conducted a systematic review and meta-analysis with the aim of assessing the association between periodontitis and CKD. This was the first review in this area and the team collected 2456 potentially relevant articles, of which nine articles were included in the review, and of those nine, four were included in the meta-analysis. The team showed that patients with periodontitis had 65% greater odds of having CKD compared with periodontally healthy patients (95% CI 35% to 101%, P < 0.00001). This implies an association between periodontitis and CKD and this association was present even after adjustment of some confounding factors that may explain the association. These confounders include shared risk factors, for example diabetes mellitus. A patient with poorly controlled diabetes is likely to have poor periodontal health30 and is likely to have decreased renal function. However, this evidence is not helpful in defining the nature of the association between periodontitis and CKD. Indeed, traditional analyses of cross-sectional surveys do not enable the direction of causality between exposure and outcome, if any, to be deduced. Hence, whether periodontal health influences renal health, or vice versa, remains uncertain. Further, a variety of measures defining periodontitis, such as varying case definitions, mean probing pocket depths, mean clinical attachment loss, number of teeth present, as well as self-reported measures of periodontitis, may have led to over- or under-estimation of the level of active disease in patients/participants examined.
In a more recent cross-sectional study of 164 Japanese individuals conducted by Naruishi et al31, the authors distinguished between diabetic and non-diabetic nephropathy and stratified these by CKD stage. The control group suffered from diabetes only. Neither periodontitis nor diabetes were clearly defined in this relatively small-scale study; however, the authors found a significant negative correlation between eGFR and the number of missing teeth, where patients with diabetic nephropathy had a higher number of missing teeth31.
In 2018, a meta-analysis was carried out with the aim to evaluate the association between periodontitis and CKD, and to explore the potential influence of periodontal treatment32. Seventeen studies were included. In their analysis, the authors took into account precise versus imprecise diagnosis of both periodontitis and CKD. Most of the identified studies indicated an increased incidence of periodontitis in patients with CKD. The strength of the association was increased in patients with severe periodontitis (OR 2.39, 95% CI 1.70 to 3.36) and was also present after adjustment for major CKD risk factors (diabetes, smoking and hypertension) or use of precise diagnosis criteria. It was concluded that periodontitis is associated with CKD after multivariable adjustment. However, with regard to intervention studies, differences in study design, absence of control groups and heterogeneity of evaluated outcomes did not allow for performing a meta-analysis of these data. Also in 2018, two other meta-analyses of observational studies with different designs confirmed the above results33,34. Zhao et al33 included 47 studies and reported substantial evidence on the non-directional association between periodontitis and CKD. In the Kapellas et al34 study, moderate evidence for a positive association between periodontitis and CKD was demonstrated, with an OR of 1.60 (95% CI 1.44 to 1.79) for periodontitis patients having CKD and an OR of 1.69 (95% CI 0.84 to 3.40) for CKD patients of having periodontitis. However, the authors of both meta-analyses point out that the high heterogeneity of the included studies limits the significance of results. Zhang et al18 further reported that the majority of the studies was of low quality. Interestingly, they also emphasised that none of the studies focused on the directional association of CKD as the exposure with periodontitis as the outcome, and only few studies on the directional association of periodontitis as the exposure with CKD as the outcome. Importantly, prospective directional association studies would draw a more precise picture of a possible cause–effect relationship.
3.2.3 Longitudinal studies exploring the relationship between periodontitis and CKD
Simplistically, longitudinal studies have the advantage over cross-sectional studies of being able to establish a temporal association, i.e. which disease came first, periodontitis or the decline in renal function. By ascertaining the preceding disease, causal assumptions can be strengthened or discarded. For example, if periodontitis preceded the commencement of CKD, the latter is unlikely to have been a cause of periodontitis.
One such study was conducted by Grubbs et al35, who followed a group of 761 elderly men (mean age over 73 years) who did not have CKD, defined by an eGFR > 60 ml/min/1.73 m35. These men were followed up for just under 5 years on average and, in that time, 56 (7.4%) developed CKD. In this study, men with severe periodontitis had a two-fold greater incidence of CKD (incident rate ratio [IRR] 2.01, 95% CI 1.21 to 3.44, P = 0.007), compared with patients who did not have severe periodontitis. This was after adjusting for confounders such as age, ethnicity, hypertension, education, smoking and diabetes status. In this study, severe periodontitis was defined in accordance with the European Workshop criteria36, as proximal clinical attachment loss ≥ 5 mm affecting 30% or more of teeth examined. This study suggests that severe periodontitis may be associated with a raised incidence of CKD. A limitation of this study is that it was conducted in elderly, predominantly white men, thereby limiting the generalisability of the findings to women, younger men or individuals from other ethnicities. The study also highlights the need for caution in the use of case definitions to define the ‘exposure’ of interest, in this case severe periodontitis, in relation to the ‘outcome’ of interest, in this case incident CKD. This is because the association with incident CKD existed when severe periodontitis was defined using the European Workshop criteria but not when severe periodontitis was defined using the CDC/AAP criteria (Centers for Disease Control/American Academy of Periodontology). The issue of defining an appropriate exposure is discussed further in Section 3.3 ‘Cellular and molecular mechanisms’.
Another longitudinal study by Grubbs et al37 examined 699 African Americans who did not have CKD (eGFR < 60 ml/min/m2). After a mean follow up of 5 years, there was an increased incidence of CKD in participants with severe periodontitis, as defined by the CDC/AAP classification of 200738, compared with participants with non-severe periodontitis (adjusted IRR 4.18, 95% CI 1.68 to 10.39). This study addresses some of the criticisms of Grubbs’ previous longitudinal study mentioned here in that it was not limited to Caucasian men. However, the small numbers of participants, coupled with a short follow-up period, led to only 21 cases of incident CKD. This makes the interpretation of these findings difficult and the findings themselves difficult to generalise.
In reality, establishing such temporal relations is not straightforward. For example, if a disease occurred before an exposure, the disease may still be caused by this exposure if the exposure is not correctly recorded. In a simple tobacco smoking/lung cancer example, if the researcher monitored only cigarette smoking, whereas the population under investigation smoked pipes, the researcher would not have measured the exposure (tobacco smoking) correctly. Furthermore, it may be that the exposure does not cause the disease in all/any patients but rather hastens the progression of the disease. Such relationships can also be gleaned from some longitudinal studies.
One study, published in 2017, which shared that aim was conducted by Chang et al39. Researchers followed 2831 patients from January 2002 to June 2013 and showed that periodontal probing pocket depths (a measure of active periodontal inflammation) and glycated haemoglobin (HbA1c) levels (a measure of the average plasma glucose concentration over a longer time span) were associated with progression of CKD (hazard ratio [HR] 3.1, 95% CI 2.0 to 4.6 for periodontal pocket depths > 4.5 mm; and HR 2.5, 95% CI 1.1 to 5.4 for HbA1c > 6.5%, respectively). Thus, patients with CKD who had worse periodontal health were more likely to experience a worsening of their kidney function compared with patients with CKD who were periodontally healthy, independent of HbA1c levels. This implies that periodontitis may be a risk factor in the progression of CKD. This important study shares one of the limitations of earlier, cross-sectional studies in that the periodontal health was only measured at one time-point and was assumed not to have changed for the duration of the trial.
Another study conducted in Japan40 followed 388 community dwelling elderly adults (aged 70 or more) for a period of 4 years. At baseline, the prevalence of CKD was 28% and over the 4-year period, the progression of attachment loss was more in participants with CKD than those without (adjusted odds ratio: 1.73; 95% CI 1.15 to 2.60). This shows the potential impact of reduced kidney function on periodontal health, thereby strengthening the potential bi-directional relationship between periodontitis and CKD.
Contrary to the studies mentioned previously, a more recent longitudinal study did not find an association between periodontitis and decreased kidney function41. This study was conducted on a relatively young (aged 20 to 59) and healthy population without CKD (baseline eGFR 118 ml/min/m2). The study examined a large cohort, over 2000 patients at baseline, and included detailed dental and medical records allowing for a robust analysis of the data. Given the relatively young age and health of these participants, the findings of the study are perhaps not surprising. During the 11-year follow-up period, the mean eGFR for the cohort changed from 118 to 105 ml/min/m2 and the percentage of participants with eGFR < 60 ml/min/m2 changed from 0.3% to 0.7%. Given the relatively low numbers of participants with CKD (both prevalent and incident), the results of this study are not generalisable to patients with CKD or those at risk of developing CKD.
3.2.4 Interventional studies investigating the association between periodontitis and CKD
Another way of determining whether periodontitis and CKD are causally linked is to test the reversibility of the association between the two disorders (i.e. to treat one disease and determine what effect this has on the other). This assumes that, if one disease contributes to the other, that contribution can be stopped or reversed by eliminating the exposure. As periodontal inflammation can be controlled by a combination of mechanical therapy and behaviour change in relation to oral hygiene, interventional clinical trials in which periodontitis is treated and the subsequent effects on renal health have been measured are more prevalent. This methodology, unfortunately, does not rule out the effect that CKD might have on periodontal health. An ideal method to test causality would be to perform an experimental study inducing periodontitis in a group of individuals and monitor whether more cases of CKD developed, compared with a control group. Due to the obvious ethical implications, these types of studies are generally conducted in animals, but are clearly undermined by the lack of an experimental model of periodontitis resembling the naturally occurring disease in humans.
The gold standard for clinical trials is the RCT, where individuals with CKD and periodontitis are randomly divided into two groups, which differ only in the intervention that they receive to manage periodontitis. The intervention may be a different method of improving periodontal health, such as providing more intensive treatment in the intervention arm and less intensive treatment in the control arm, or deferring the periodontal intervention in the control arm for a period of time, generally for 3, 6 or 12 months. In non-RRT CKD patients, no such trials have currently published results, but there are three trials for which published trial protocols are available42–44.
Table 3-4 Interventional studies evaluating the effect of periodontal therapy in pre-dialysis chronic kidney disease (CKD)
| Study | Duration | Patient number | Marker evaluated | Results |
|---|---|---|---|---|
| Artese et al45 | 3 mo | Patients with periodontitis: 21 pre-dialysis CKD and 19 without CKD | eGFR, serum creatinine | eGFR improved in both groups. Patients with CKD mildly significantly improved from 46.5 to 50.7 ml/min/1.73 m2 and patients without CKD significantly improved from 91.7 to 105.3 ml/min/1.73 m2. No significant improvement in serum creatinine levels was found following periodontal therapy |
| Almeida et al46 | 6 mo | 26 patients with severe periodontitis and CKD | eGFR, triglycerides, total cholesterol, albumin, asymmetric dimethylarginine | Mildly significant improvement on the median values of eGFR (from 34.6 to 37.6 ml/min/1.73 m2) and of asymmetric dimethylarginine levels. No significant differences in metabolic marker concentrations |
| Vilela et al47 | 3 mo | Patients with periodontitis: 36 with pre-dialysis CKD and 20 without CKD | Prohepcidin, IL-6, us-CRP | Prohepcidin, IL-6, and us-CRP levels decreased significantly after PT in both groups |
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; IL-6 = interleukin 6; PT = periodontal therapy; us-CRP = ultra-sensitive C-reactive protein.
Several trials, most of them non-randomised, have been conducted in this field (Table 3-4). One study45, which hypothesised that patients with CKD may not respond to periodontal therapy due to being immunocompromised, found to the contrary, that periodontal therapy was successful in such patients. This study was performed on patients with periodontitis (21 pre-dialysis patients and 19 patients without clinical evidence of kidney disease) but was underpowered to show an improvement in renal function. The results were supported by a pilot study recently conducted by Almeida et al46; however, this study had a similar population size and did not include a control group. Another study47 carried out in patients undergoing dialysis found that periodontal therapy was associated with significant reductions in systemic inflammatory biomarkers including high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6) and serum prohepcidin levels. Moreover, Guo and Lin48, in a 2017 study, demonstrated that CKD patients with periodontitis had decreased systemic levels of hsCRP, IL-6 and TNF-α after periodontal treatment. A further independent study49 demonstrated that periodontal therapy may have a beneficial effect on one marker of renal function (cystatin C). However, this study was conducted on 20 systemically healthy volunteers, and the results may not be applicable to patients with established CKD. In an RCT, published in 2018, on patients suffering from periodontitis and type 2 diabetes mellitus, but not necessarily CKD, intensive periodontal therapy (subgingival debridement including surgical periodontal therapy, if eligible, and supportive periodontal therapy every 3 months until completion of the study) was associated with an improvement of kidney function compared to patients who received only supragingival scaling and polishing of their teeth over a 12-month period50. The improvement of kidney function was also associated with improved metabolic control, vascular function and reduced systemic inflammatory burden. As this study did not include patients with established CKD, the effects of periodontal therapy on renal function in such patients cannot be surmised.
SUMMARY
● There is robust evidence from observational studies and meta-analyses, of the association between periodontitis and mortality in patients with CKD.
● There is robust evidence from cross-sectional studies and meta-analyses, of the association between periodontitis and CKD.
● There is limited evidence from prospective longitudinal studies, of the effect of periodontitis on decline in renal function.
● There is very limited evidence from RCTs, of the effect of treatment of periodontitis on improvements in the systemic health of patients with CKD.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


