Fig. 6.1
(a, b) Biologic width – tooth versus implant: diagrammatic schematics of microscopical aspect of the peri-implant tissue in relation to the periodontal tissues
It has been shown by studies conducted from the early 1990s to the early 2000s with external hex design that the mucosal barrier surrounding dental implants is formed by a crevicular oral epithelium ranging in mean 1.5–2 mm (but it may reach up to 4 mm) and a connective tissue barrier in mean ranging 1–2 mm [6–8]. Moreover, the oral epithelium presents an extension with a thin junctional epithelium facing the implant surface and extending about 1.64–2.35 mm from the mucosal margin (Fig. 6.1, 6.2, and 6.3) [6–8]. In general terms, the length from the marginal portion of the peri-implant mucosa to the first bone-to-implant contact has been found to be approximately 3 mm [6–8].
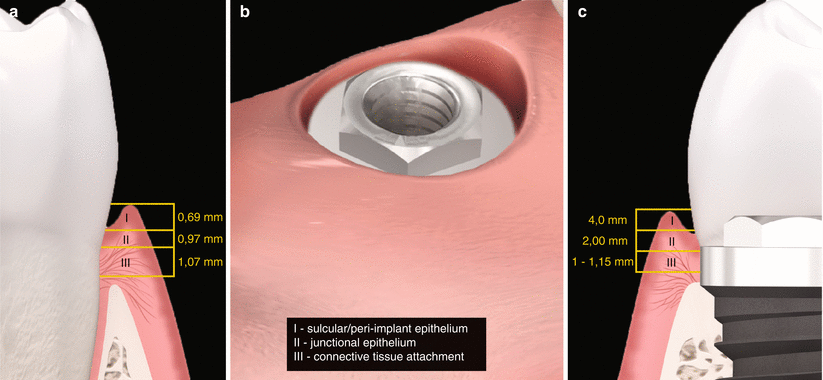
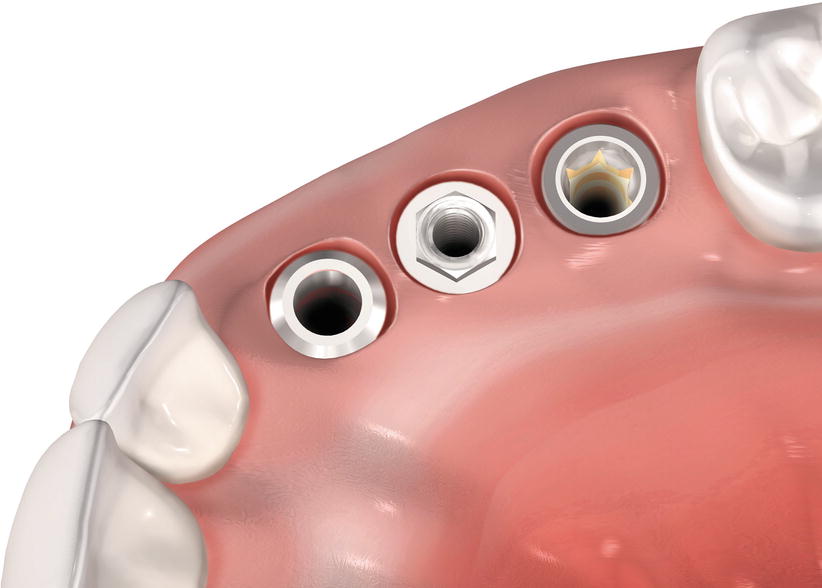

Fig. 6.2
(a–c) Biologic width of implants compared to natural teeth

Fig. 6.3
Profile of the soft tissue mucosa at different types of implants
In addition, it was demonstrated that a 2 mm height saucer-shaped crestal bone resorption occurs (a phenomenon called as “saucerization”) when submerged, 2-piece implants are used based on the location of the microgap, whereas for non-submerged, 1-piece implants, none or minimal loss may occur (Fig. 6.4) [9, 10]. It is also important to note for the 2-piece implants that such a bone change only occurs after the period of osseointegration/healing (submerged healing phase), within the first month following the placement of abutments [10]. The specific mechanisms linked to this crestal bone remodeling in 2-piece implants are not completely known [5], but these have been related in some animal model studies to microbial colonization of the microgap, micro-movements of the abutment, or an interruption of the blood supply when implants and abutments are placed transmucosally [10–12].
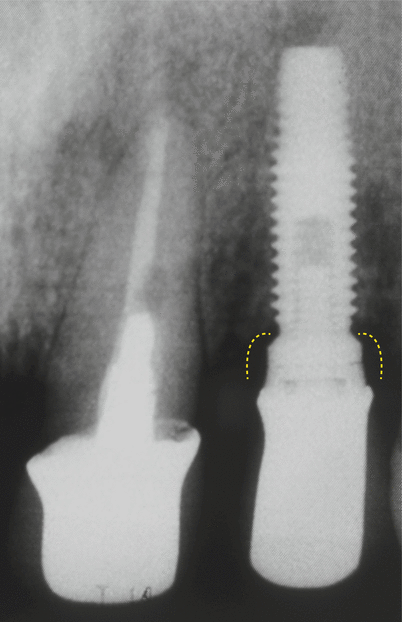

Fig. 6.4
Saucerization around an external hex implant
Around dental implants, BW determines the minimum dimensions to ensure junctional epithelium and connective tissue to attain an ideal seal and to provide protection from mechanical and external biological agents [13]. Also, an external agent invading the BW would induce a response from the epithelium that migrates beyond this agent trying to isolate it [2, 3, 13]. The resulting bone resorption produces a reestablishment of the BW dimension.
In humans, recent data showed that the biologic width around one- and two-piece retrieved implants is formed by (1) a crevicular epithelium composed of 4–6 layers of parakeratinized epithelial cells; (2) a junctional epithelium formed by 5–10 layers of epithelial cells, where the middle and apical portion of the JE consisted of 3–5 cells layers; and (3) a connective tissue (at the abutment area) containing few blood vessels (only from the supraperiosteal plexus), dense collagen fibers, and reduced number of fibroblasts (when compared to the lamina propria of the gingiva) oriented parallel to the longitudinal axis of the abutment [14]. Regarding to the dimensions of the biologic width around these implants, the following can be expected (Fig. 6.5):
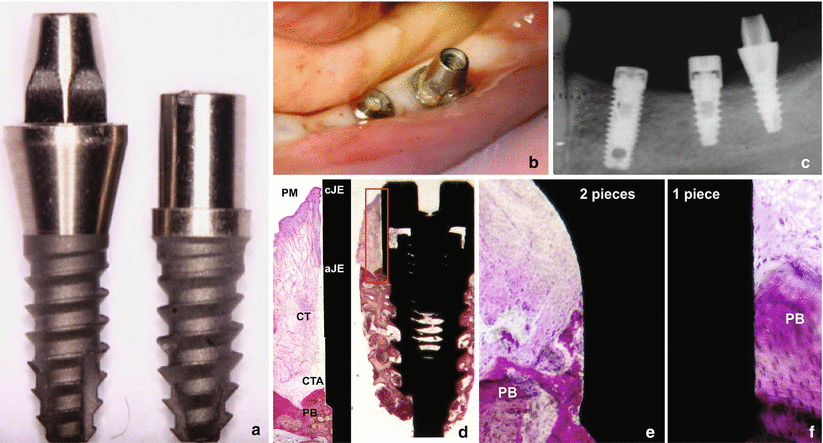

Fig. 6.5
Clinical, radiographic, and photomicrograph view of the biologic width around implants installed in human jaw: (a) one- and two-piece implants, (b) clinical, (c) radiographic, (d) photomicrograph of the ground section of the two-piece implant (20×) and the high magnification of the biologic width (100×), and (e, f) photomicrographs of the one- and two-piece implant (200×) near to the first bone to implant contact. Note the disorganization around the peri-implant bone close to the microgap on the two-piece implant. PM perimplant mucosa, PB perimplant bone, CTA connective tissue attachment, CT connective tissue, CJE most coronal point of junctional epithelium, AJE most apical point of junctional epithelium (Figure originally published at Judgar et al. [14])
In addition, the biologic width formation and maturation around dental implants take place between 6 and 8 weeks of wound healing, and the connective tissue of the implant mucosa is similar in composition (cells, fiber orientation, and vascularization) to a scar tissue [16].
6.3 Type of Defect to Be Indicated
Peri-implant sites requiring keratinized tissue width and thickness gain due to aesthetical and/or functional purposes, aesthetical treatment of alveolar ridges presenting osseous concavities or deformities, treatment of peri-implant mucosa recession, and alveolar ridge preservation (in association to bone graft materials) (Figs. 6.6, 6.7, 6.8, 6.9, 6.10, 6.11, and 6.12).
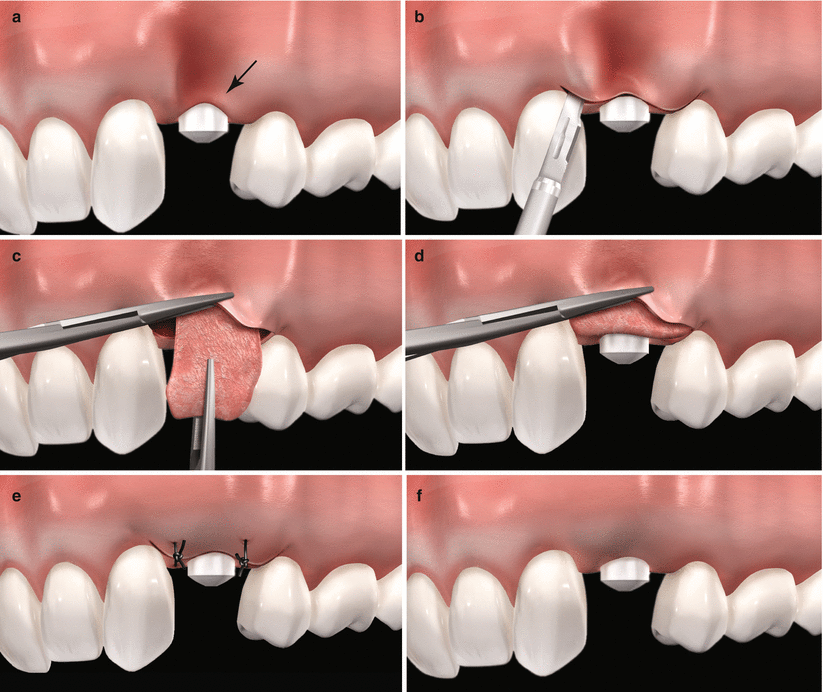
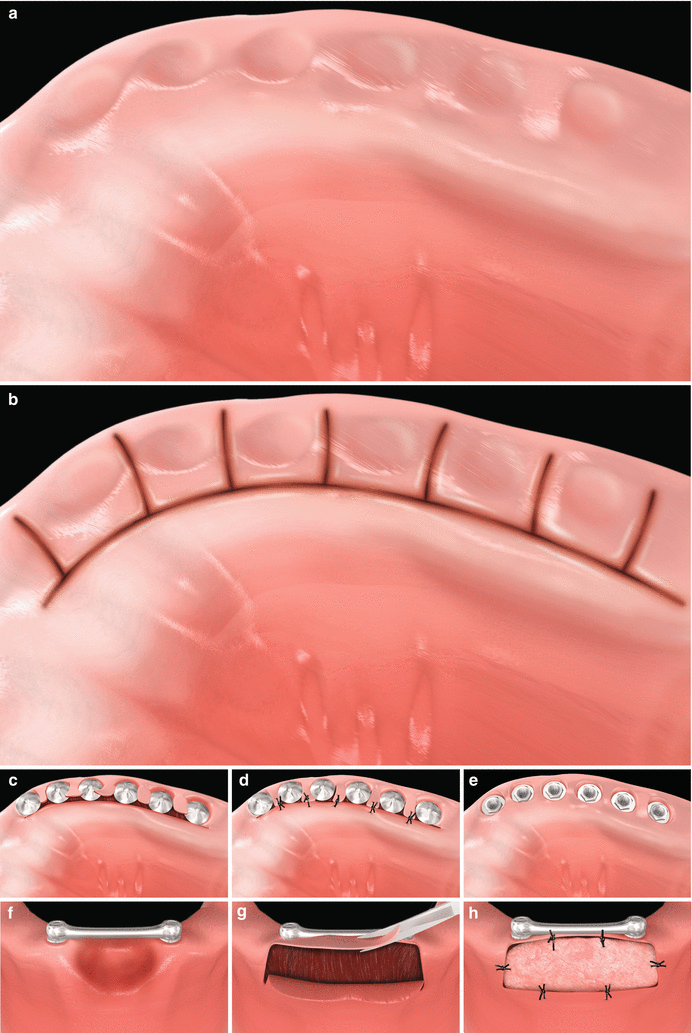
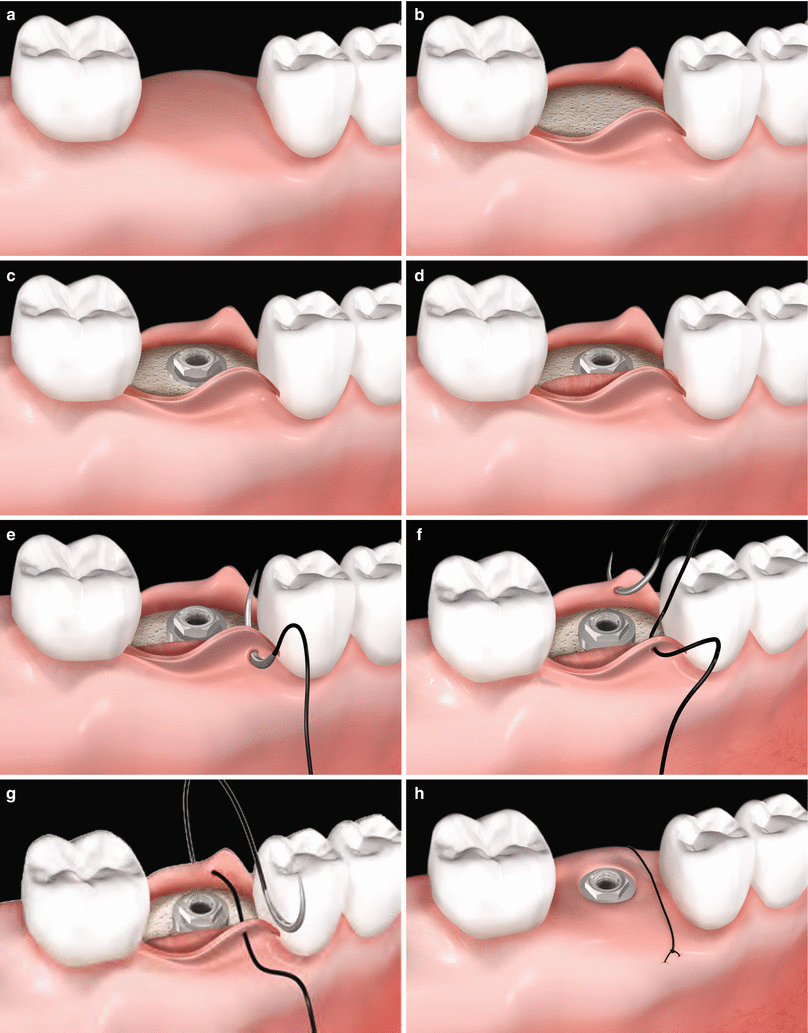
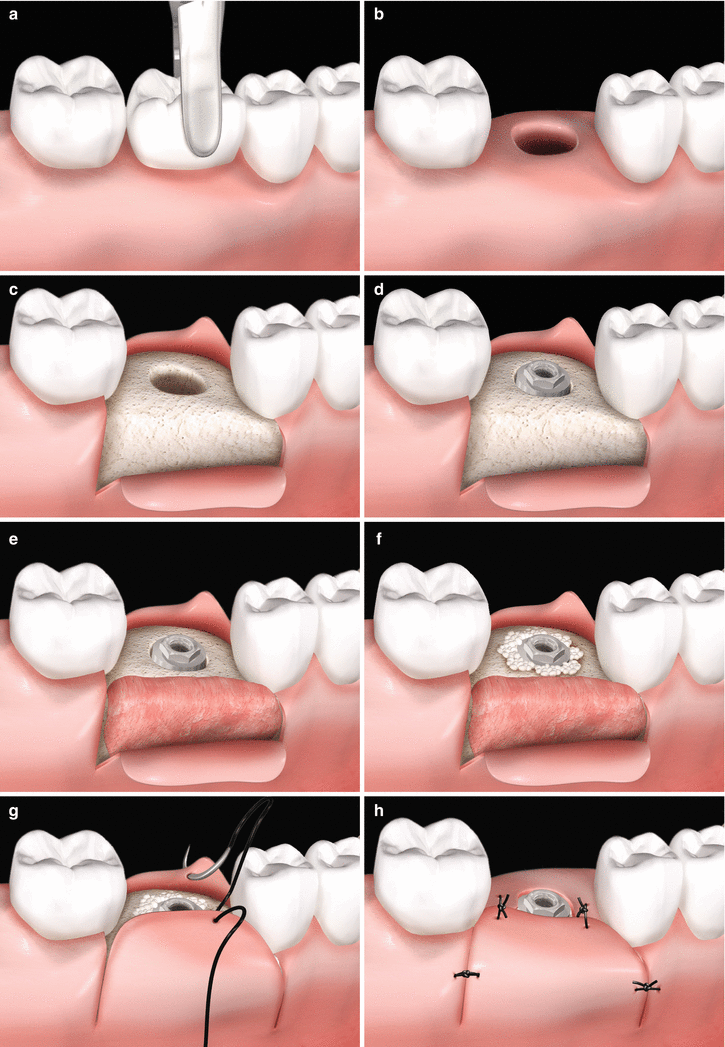
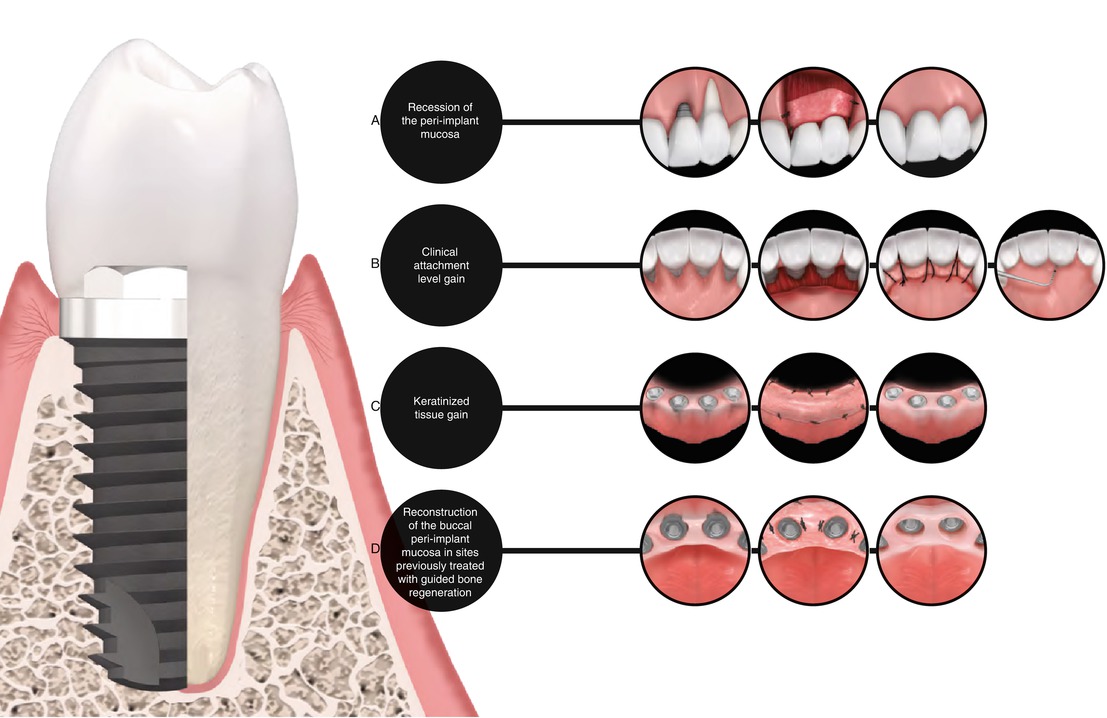
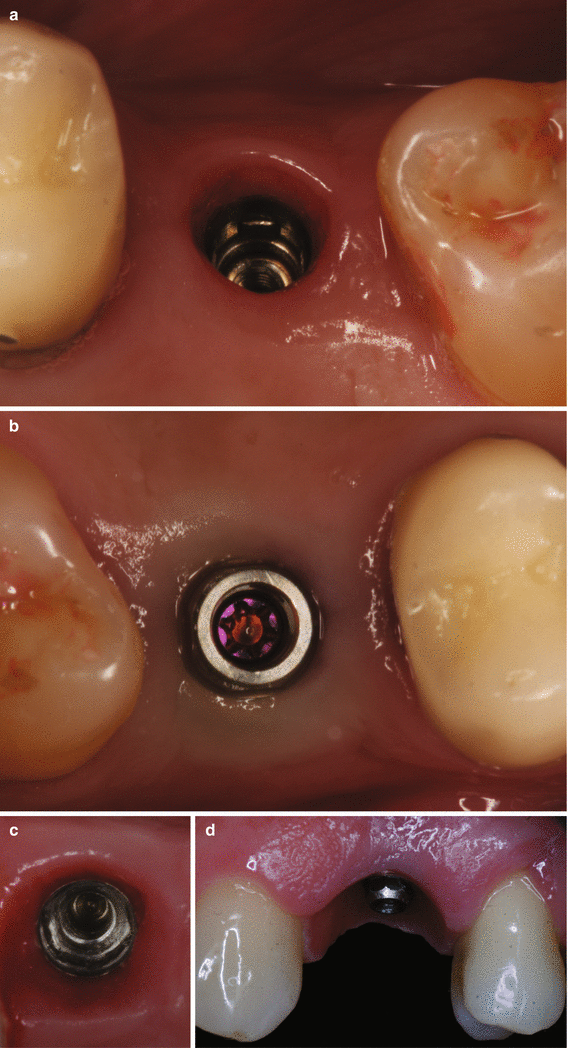
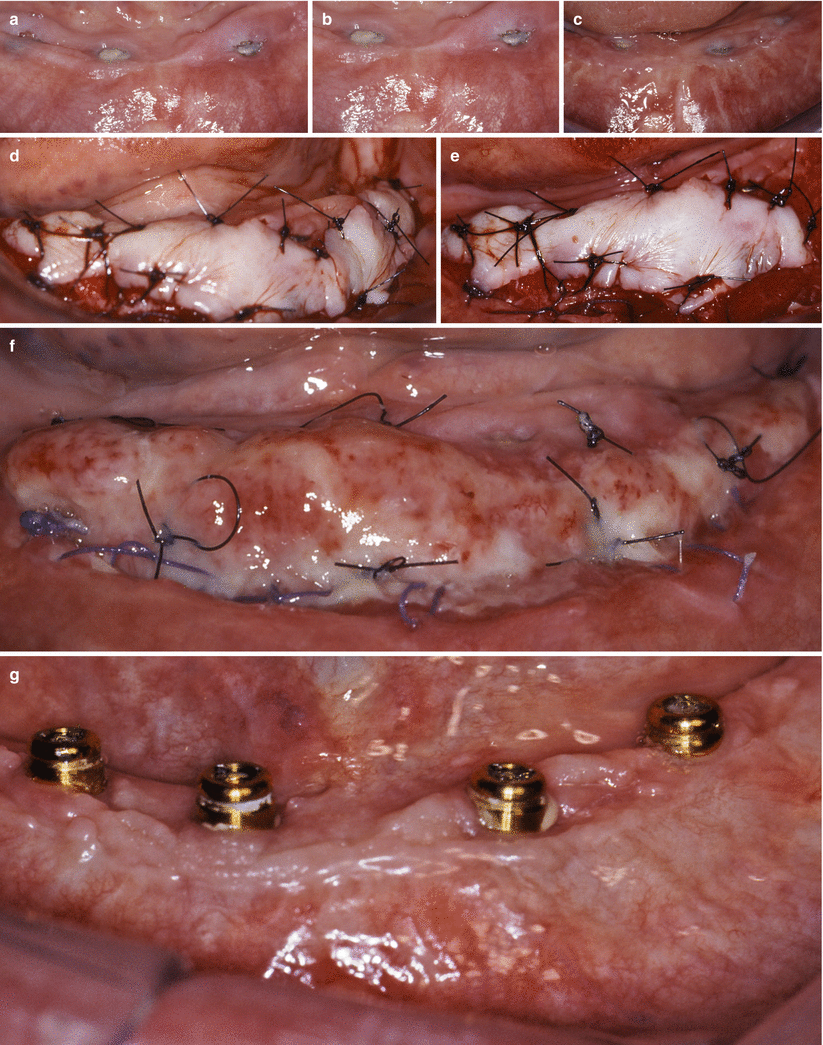

Fig. 6.6
Schematic representation of the use of subepithelial connective tissue graft in alveolar ridges previously treated with dental implants. Note the alveolar defect associated to the buccal resorption of the ridge subsequent to tooth extraction, (a) intrasulcular incision and rising of a partial-thickness flap, (b) placement of the graft through the envelope flap, (c) graft positioning 1–2 mm coronary to the peri-implant osseous ridge, (d) flap sutured covering completely the graft (e) expect outcome (f)

Fig. 6.7
Reopening procedures (a–e). Alveolar ridge with submerged implants (a). Palatal and interproximal incisions (b). Lateral positioning of the pedicle flaps (c). Suture of the pedicle flaps (d). Expected outcome (e). Removal of a soft tissue hyperplasia and gain in the keratinized tissue with free gingival graft around dental implants supporting overdentures (f–h). Excision of the hyperplasia and preparation of the recipient site for free gingival grafting (f, g). Free gingival sutured at the recipient site (h)

Fig. 6.8
(a–h) Soft tissue grafting concomitant to implant placement. Edentulous alveolar ridge prior to implant placement (a). Full-thickness flap rising (b). Implant installation (c). Placement of a soft tissue graft (d). Sequence of suture (e–h)

Fig. 6.9
(a–h) Soft tissue grafting concomitantly to implant placement – fresh extraction site. Tooth to be removed (a). Osseous cavity after tooth extraction (b). Full-thickness flap rising (c). Implant placement (d). Positioning of a soft tissue graft (e). Placement of bone substitute filling the gap between the implant surface and the bone walls at the extraction site (f). Suture sequence (e–h)

Fig. 6.10
(a–d) Other potential indications for peri-implant plastic surgery

Fig. 6.11
(a–d) Healthy peri-implant mucosa

Fig. 6.12
Keratinized tissue gain with free gingival grafts prior the fabrication of overdenture. Lack of keratinized tissue around implants (a–c). Free gingival graft sutured (d, e). Ten-day follow-up (f). Two-year follow-up (g)
6.4 Type of Defect Not to Be Indicated
None, but predictability may vary according to the severity of the defect/deformity, as well as the biomaterial used.
6.5 Clinical Remarks: Implications for Practice and Clinical Decision-Making of Peri-implant Defects and Conditions
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


