The ideal management of peri-implant diseases focuses on infection control, detoxification of implant surfaces, regeneration of lost tissues, and plaque-control regimens via mechanical debridement (with or without raising a surgical flap). However, a variety of other therapeutic modalities also have been proposed for the management of peri-implantitis. These treatment strategies encompass use of antiseptics and/or antibiotics, laser therapy, guided bone regeneration, and photodynamic therapy. The aim of this article was to review indexed literature with reference to the various therapeutic interventions proposed for the management of peri-implant diseases.
Key points
- •
Risk factors of peri-implant mucositis and peri-implantitis are comparable to those of gingivitis and periodontitis.
- •
The ideal management of peri-implant diseases focuses on infection control, detoxification of implant surfaces, regeneration of lost tissues, and plaque control regimens via mechanical debridement.
- •
Implantoplasty (modification in implant surface topography), when used in combination with resective surgery, has been reported to significantly reduce the clinical parameters of peri-implantitis.
- •
A new technique, laser-assisted peri-implantitis protocol, is under investigation.
- •
There is lack of standardized treatment protocols for peri-implant disease.
Several studies have reported that dental implants are functionally stable and have long-term success rates, and are therefore increasingly being used in the oral rehabilitation of partially and completely edentulous individuals. However, with the increasing number of patients receiving dental implants, the prevalence of inflammatory conditions around a dental implant has also escalated. The consensus report from the 6th European Workshop on Periodontology has defined peri-implantitis as the presence of inflammation of the peri-implant mucosa and simultaneously loss of supporting bone. In addition, it has also been described as a site-specific infection that exhibits features comparable to those of chronic adult periodontitis. In indexed literature, there is a variance in the prevalence of peri-implantitis. Mombelli and colleagues estimated the prevalence of peri-implantitis to be in the order of 10% implants and 20% patients during 5 to 10 years after implant placement; and Koldsland and colleagues reported the prevalence to range between 11.3% and 47.1% in their study population. Moreover, in a recent study on Belgian adults, the prevalence of peri-implant mucositis and peri-implantitis was 31% and 37%, respectively. It has been suggested that peri-implant mucositis and peri-implantitis are analogous to gingivitis and periodontitis, respectively. However, it is pertinent to mention that biologic differences exist between natural teeth and implants. Therefore, the progression of infection around implants and natural teeth is also divergent. Moreover, tissues around implants are more prone to plaque-associated infections that spread into the alveolar bone.
The ideal management of peri-implant diseases focuses on infection control, detoxification of implant surfaces, regeneration of lost tissues, and plaque-control regimens via mechanical debridement (with or without raising a surgical flap). However, a variety of other therapeutic modalities also have been proposed for the management of peri-implantitis. These treatment strategies encompass use of antiseptics and/or antibiotics, laser therapy, guided bone regeneration, and photodynamic therapy. The aim of this article was to review indexed literature with reference to the various therapeutic interventions proposed for the management of peri-implant diseases.
Peri-implant diseases: peri-implant mucositis and peri-implantitis
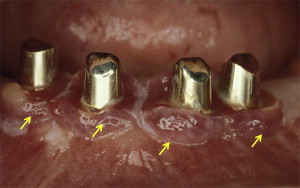
Peri-implant diseases are categorized into 2 types: peri-implant mucositis and peri-implantitis. Peri-implant mucositis is characterized by inflammation of the soft tissues surrounding the implant without any signs of bone loss ( Fig. 1 ). The clinical signs of peri-implant mucositis include bleeding on probing (BOP) and/or suppuration, which are usually associated with probing depth (PD) of at least 4 mm with no evidence of radiographic loss of bone. It has been reported that inflammatory cell lesions in sites with peri-implant mucositis are dominated by T cells and have an apical extension that is restricted to the barrier epithelium. Peri-implant mucositis is usually reversible (when early diagnosis and removal of etiology are implemented) ; however, it is considered as a precursor to peri-implantitis.
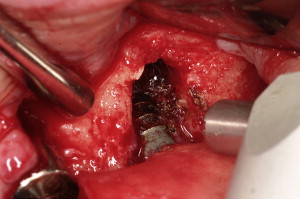
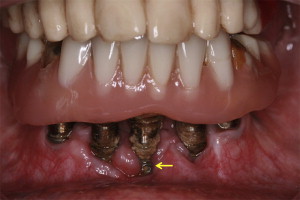
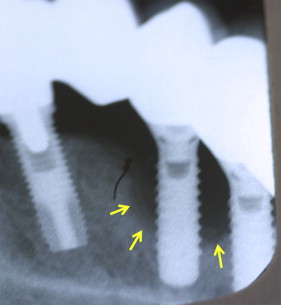
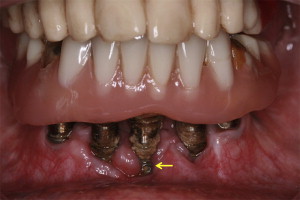
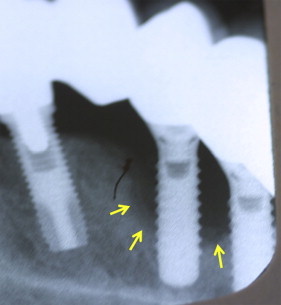
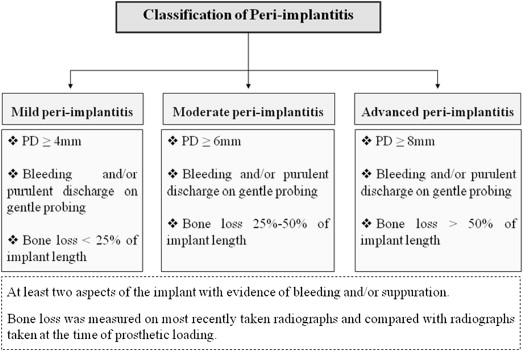
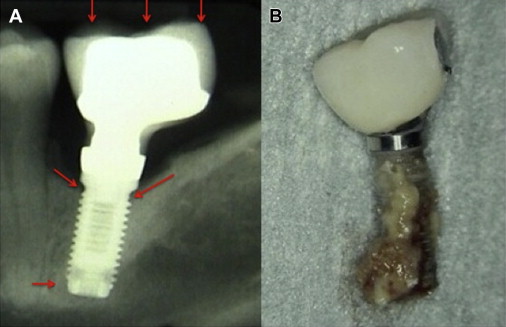
The term “peri-implantitis” was introduced in literature more than 3 decades ago. This term was modified in the 1990s to describe an inflammatory process around an implant that includes both soft tissue inflammation and progressive loss of supporting bone beyond biological remodeling ( Figs. 2 and 3 ). In general, the clinical definition of peri-implantitis has varied among studies. For example, in studies by Roos-Jansåker and colleagues, peri-implantitis was described as a condition in which implants with varying degrees of bone loss are accompanied by a PD of at least 4 mm, BOP, and purulent discharge on gentle probing. However, in a systematic review by Berglundh and colleagues, peri-implantitis was defined by PD of more than 6 mm and in combination with BOP and clinical attachment loss or bone loss of at least 2.5 mm ( Fig. 4 ).
Peri-implant diseases: peri-implant mucositis and peri-implantitis
Peri-implant diseases are categorized into 2 types: peri-implant mucositis and peri-implantitis. Peri-implant mucositis is characterized by inflammation of the soft tissues surrounding the implant without any signs of bone loss ( Fig. 1 ). The clinical signs of peri-implant mucositis include bleeding on probing (BOP) and/or suppuration, which are usually associated with probing depth (PD) of at least 4 mm with no evidence of radiographic loss of bone. It has been reported that inflammatory cell lesions in sites with peri-implant mucositis are dominated by T cells and have an apical extension that is restricted to the barrier epithelium. Peri-implant mucositis is usually reversible (when early diagnosis and removal of etiology are implemented) ; however, it is considered as a precursor to peri-implantitis.
The term “peri-implantitis” was introduced in literature more than 3 decades ago. This term was modified in the 1990s to describe an inflammatory process around an implant that includes both soft tissue inflammation and progressive loss of supporting bone beyond biological remodeling ( Figs. 2 and 3 ). In general, the clinical definition of peri-implantitis has varied among studies. For example, in studies by Roos-Jansåker and colleagues, peri-implantitis was described as a condition in which implants with varying degrees of bone loss are accompanied by a PD of at least 4 mm, BOP, and purulent discharge on gentle probing. However, in a systematic review by Berglundh and colleagues, peri-implantitis was defined by PD of more than 6 mm and in combination with BOP and clinical attachment loss or bone loss of at least 2.5 mm ( Fig. 4 ).
Risk factors of peri-implant diseases
Risk factors of peri-implant mucositis and peri-implantitis are comparable to those of gingivitis and periodontitis. The following risk factors have been associated with the etiology of peri-implant diseases.
Poor Plaque Control
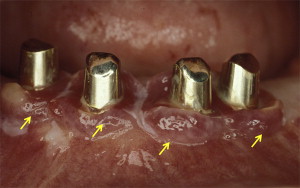
Implant geometry and surface characteristics may obligate patients to adopt nonconventional plaque-control regimens, such as interdental brushing and flossing in addition to traditional tooth-brushing techniques. The primary focus of these nonconventional plaque-control protocols is to maintain the periodontal health of the remaining dentition, thereby minimizing the possibilities of plaque accumulation and formation of periodontal and/or peri-implant pockets. However, it may be challenging for some patients to get accustomed to nonconventional plaque-control regimens (as stated previously) without ado. This may in turn compromise their ability in executing plaque control (see Fig. 2 ). In this regard, it is imperative for dental health care providers to educate and regularly monitor their patients (through routine check-ups) to ensure proper plaque control. This would in turn help patients in maintaining their periodontal and/or peri-implant health.
Previous History of Periodontal Disease
It has been claimed that peri-implantitis is a common finding in patients with a history of periodontitis. Results from a systematic review and meta-analysis showed that the relative risk for peri-implantitis was significantly higher in patients with a previous history of periodontitis compared with patients without a history of periodontal disease. However, in a recent study, Meyle and colleagues investigated the long-term clinical and radiographic parameters of osseointegrated implants in nonsmoking patients with a previous history of chronic periodontitis. The results showed that patients with a previous history of periodontitis regularly attending an oral hygiene maintenance program displayed implant survival rates up to 100% after 5 and 10 years. Similarly, in a systematic review, Pesce and colleagues concluded that there is a lack of consensus regarding the role of periodontitis in the etiology of peri-implantitis. Nevertheless, because several periodontopathogens (such as Aggregatibacter actinomycetemcomitans , Prevotella intermedia , and Porphyromonas gingivalis ) associated with the etiology of periodontitis also have been isolated from peri-implant sulci of patients with peri-implantitis, it is arduous to disregard the hypothesis that peri-implantitis is more common in patients with a history of periodontitis.
Stagnation of Residual Material in/Around the Gingivae Following Implant Prosthesis Cementation
A conventional approach toward restoration of dental implants using fixed prosthesis is the use of cement-retained restorations. In the absence of occlusal screw access openings, cement-retained restorations are useful in enhancing the number of occlusal contacts and simultaneously improving aesthetics. However, inadequate removal of excessive cement at the time of implant cementation may lead to a complication: cement-induced peri-implantitis. The probability of cement to remain in the peri-implant sulcus is high when margins of the restoration are placed 1.5 to 3 mm subgingivally. Stagnation of residual cement in peri-implant sulcus has been associated with peri-implant tissue inflammation, BOP and/or suppuration, increased PD, evidence of bone loss on radiographs, and patient discomfort. Shallow intracrevicular placement of the gingival margins reduces the occurrence of peri-implant inflammation and related complications.
Occlusal Overloading
Occlusal overloading is a major cause of biomechanical implant complications, including fracture and/or loosening of the implant ( Fig. 5 ). Occlusal overloading in combination with plaque accumulation may also disturb the intricate bond between the implant surface and bone leading to peri-implantitis and, if left untreated, implant failure. However, conflicting results also have been reported. In a study on dogs, there was no loss of osseointegration and/or peri-implantitis following a period of 8 months of excessive occlusal load on titanium (Ti) implants. Occlusal overloading may be prevented by performing comprehensive examinations, treatment planning, well-defined surgical and prosthetic treatments, and regular maintenance. However, Pesce and colleagues reported in a recent systematic review that there is a lack of clinical evidence to support a cause-effect relationship between peri-implantitis and occlusal overload.
Habitual Tobacco Smoking
Tobacco smoking is a significant risk factor for periodontitis and peri-implant diseases, and pathogenesis of periodontitis closely resembles that of peri-implantitis. Tobacco smoking has been associated with an increased expression of receptor of advanced glycation end products (RAGE) in gingival tissues. Experimental results by Katz and colleagues showed an increased expression of RAGE in gingival epithelial cells of smokers as compared with nonsmokers. Moreover, it also has been reported that nornicotine (a metabolite of nicotine) upregulates RAGE expression in the gingivae of smokers. This stimulates the secretion of cytokines and reactive oxygen species, which enhance alveolar bone loss. Furthermore, there is a synergistic effect of tobacco smoking and carriage of interleukin (IL)-1 gene polymorphism that results in increased risk of peri-implantitis.
Galindo-Moreno and colleagues reported that the rates of marginal bone loss around implants are significantly associated with smoking. Studies have reported that rates of implant failure range between 6.5% and 20.0% among smokers compared with nonsmokers. In smokers, implant failures occur more often in the maxilla as compared with implants placed in the mandible ; most likely due to the presence of poor-quality trabecular bone in the maxilla. Results from a recent systematic review and meta-analysis also reported a significantly higher risk of peri-implantitis in smokers as compared with nonsmokers.
Diabetes
It is well acknowledged that periodontal inflammatory conditions are worse in patients with poorly controlled diabetes as compared with systemically healthy individuals. This is primarily because chronic hyperglycemia impairs tissue repair and host defense mechanisms. In addition, an increased formation and accumulation of advanced glycation end-products in the periodontal tissues raises cellular oxidative stress and increases the production of proinflammatory cytokines (such as IL-6, IL-1β, IL-18, matrix metalloproteinase [MMP]-8 and MMP-9) in the serum, saliva, and gingival crevicular fluid of patients with chronic hyperglycemia. These proinflammatory cytokines may jeopardize the osseointegration and long-term survival of implants in patients with chronic hyperglycemia. However, it is pertinent to mention that degree of glycemic control (in patients previously diagnosed with diabetes) may play a role in a successful osseointegration and survival of implants in diabetic patients.
Genetic Factors
A correlation between IL-1 gene polymorphism and peri-implantitis has been reported by some studies. However, in a systematic review, Dereka and colleagues reported no apparent association between specific genetic polymorphism and dental implant failure in terms of biological complications (peri-implant diseases). Hultin and colleagues, Rogers and colleagues, and Campos and colleagues also reported no association between gene polymorphism and peri-implantitis. Although the association of genetic factors with peri-implantitis cannot be completely disregarded, further studies are warranted to assess the role of genetics as a potential risk factor of peri-implantitis.
Incidence and prevalence of peri-implant diseases
There is a lack of information regarding the incidence of peri-implant mucositis and peri-implantitis, as the interpretation of data is difficult. In this regard, the incidence of peri-implantitis may be underestimated. Renvert and colleagues investigated the incidence of peri-implantitis over a period of 13 years between 2 dental implant systems (TiOBlast AstraTech [AT] and machine-etched Brånemark Nobel Biocare [NB] system, Gothenburg, Sweden, respectively). In this study, the incidences of peri-implantitis for AT implants were 26.2% and 7.1% between years 1 and 7; and 30.4% and 11.5% for NB implants between years 7 and 13. There was no significant difference in the incidence of peri-implantitis over a period of 13 years between the 2 implant systems. The investigators considered a history of periodontitis as a risk for future incidence of peri-implantitis. The consensus report of the 7th European Workshop on Periodontology also emphasized that there is a need to develop new and more efficient preventive and treatment approaches for periodontal disease. This report also accentuated such preventive and treatment protocols should be based on recent advances in understanding of host modulation and inflammation resolution with direct management of the microbiota.
To our knowledge, only a limited number of studies have assessed the prevalence of peri-implant disease. Moreover, we observed that the prevalence reported in these studies varied between patients and implant systems. For example, in the 5-year follow-up results by Fransson and colleagues, the prevalence of peri-implant diseases was reported to be 92%, whereas in the study by Scheller and colleagues, the prevalence of peri-implant diseases at 5-year follow-up was 24%. Zitzmann and Berglundh reported the prevalence of peri-implantitis to vary between 12% and 43% of implants. This variation may be associated with a lack of standardization in the patient selection and criteria for diagnosing peri-implant mucositis and peri-implantitis among these studies. Therefore, a precise estimation of the prevalence of peri-implant disease remains debatable.
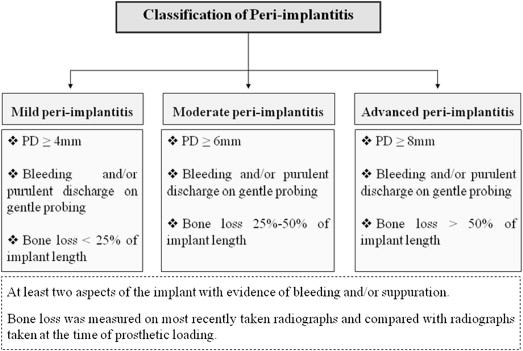
Classification of peri-implantitis
To our knowledge from indexed literature, there is no globally accepted classification of peri-implantitis. However, Froum and Rosen recently proposed a classification that differentiated peri-implantitis according the severity of inflammation and extent of bone loss around the implant. This classification was based on 3 different clinical stages of peri-implantitis: (1) early peri-implantitis, (2) moderate peri-implantitis, and (3) advanced peri-implantitis (see Fig. 4 ). Early peri-implantitis was defined by PD of at least 4 mm and radiographic bone loss of less than 25% of the total implant length. Moderate peri-implantitis was defined by PD of at least 6 mm and radiographic bone loss of 25% to 50% of the total implant length, whereas advanced peri-implantitis was defined by PD of at least 8 mm with more than 50% of radiographic bone loss with reference to implant length. In this classification, BOP and/or suppuration on 2 or more aspects of the implant were selected as the markers of inflammation. The investigators conjectured that their proposed classification would help strengthen communication between clinical practitioners and researchers and help attain a better understanding of peri-implantitis ; however, further evidence-based clinical studies are needed to authenticate this classification.
Assessment of end points among studies on the treatment of peri-implantitis
End points are commonly used to determine the effectiveness of a clinical treatment. In studies addressing the treatment of peri-implantitis, ‘‘true end points” are preferred over ‘‘surrogate end points,’’ because they are able to depict a considerable proportion of the effect of treatment on an outcome of interest, such as implant failure. In a systematic review, Faggion and colleagues explored indexed literature to determine the type of end points reported regarding peri-implantitis therapy and their frequency of use. Studies included in this systematic review chiefly reported implant failure as a consequence of peri-implantitis therapy and not as an objective of an investigation. The investigators concluded that there is a lack of consensus regarding the efficacy of treatment of peri-implantitis for reducing the risk of implant failure. A similar conclusion was reported by Lee.
Etiology and pathogenesis of peri-implantitis
Microbial colonization is the chief predisposing factor in the etiology of peri-implantitis. Subgingival microbes (such as Aggregatibacter actinomycetemcomitans, Prevotella intermedia, P gingivalis, and Treponema denticola ) associated with the etiology of periodontitis are similar to those of peri-implantitis. In addition, it also has been reported that patterns of plaque formation and accumulation on implant surfaces are comparable to those seen on teeth.
The pathogenesis of peri-implantitis is similar to that of periodontitis. Microbes in plaque stagnated on implant surface(s) release chemotactic peptides that attract neutrophils toward the peri-implant pocket(s). Moreover, cytokines are released into the peri-implant crevicular fluid as a result of microbial damage to epithelial cells, which attract more leukocytes (predominantly neutrophils) toward the affected site. If neutrophils become overloaded with microbes, they degranulate and the toxic enzymes released from neutrophils cause tissue damage and gingival inflammation. If the inflammation persists, it may progress to marginal gingiva and ultimately cause bone loss, a classical feature of peri-implantitis. Stromal cells (such as granulation tissue fibroblasts) also may participate in the pathogenesis of peri-implantitis by upregulating vascularity and matrix breakdown, thereby promoting migration/maintenance of infiltrates (proinflammatory cytokines) into the inflamed site.
Management of peri-implantitis
A variety of treatment protocols have been proposed for the management of peri-implantitis. The management of peri-implantitis can be divided into (1) nonsurgical management and (2) surgical management; surgical management encompass resective and regenerative treatment. However, there is lack of standardized treatment protocols for peri-implant disease. The following text comprehensively reviews the different therapeutic protocols that have been proposed for the management of peri-implant diseases.
Nonsurgical Management of Peri-implant Diseases
Nonsurgical (closed debridement) treatment of peri-implant disease involves the mechanical removal of plaque and calculus (using mechanical instruments, such as scalers and curettes) from the implant surfaces ( Fig. 6 ). The significance of antibiotics and/or antiseptic oral rinses (including chlorhexidine [CHX]) as adjuncts to mechanical debridement remains debatable. Renvert and colleagues systematically reviewed indexed literature that assessed the nonsurgical management of peri-implant mucositis and peri-implantitis. They reported that nonsurgical mechanical debridement alone is effective in the treatment of peri-implant mucositis. The adjunct use of antiseptic oral rinses in combination with nonsurgical mechanical debridement improved the treatment outcome of peri-implant mucositis as compared with the use of nonsurgical mechanical debridement alone. In a study on cynomolgus monkeys, Trejo and colleagues reported mechanical debridement with or without the adjunct use of CHX is effective in the treatment on experimental peri-implant mucositis. In addition, histologic results by Trejo and colleagues showed minimal inflammation in groups treated by mechanical debridement (regardless of CHX use) as compared with the control group (no treatment group). However, Schwarz and colleagues reported limited effect of nonsurgical mechanical debridement on the treatment of peri-implant diseases irrespective of type of adjunct treatment used. Similar results were reported by Karring and colleagues. Clinical results by Khoury and Buchmann showed a temporary improvement in PD following nonsurgical treatment of peri-implant diseases. This could possibly have occurred due to an incomplete decontamination of implant surfaces during the nonsurgical treatment approach.
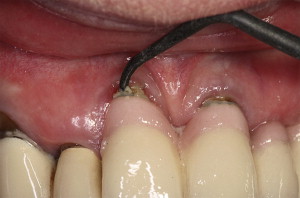
Although mechanical implant surface debridement constitutes the basic element for treatment of peri-implant diseases, other factors, such as implant positioning, geometry, and surface characteristics, may influence the overall outcomes of mechanical treatment of infected implants. There is a need for further randomized controlled trials assessing treatment modalities of nonsurgical management of peri-implant mucositis and peri-implantitis.
Surgical Management of Peri-implant Diseases
Surgical management of peri-implantitis is usually recommended in patients with severe forms of peri-implant diseases (PD >5 mm and bone loss) ( Fig. 7 ). Three-year follow-up results from a clinical trial showed that surgical treatment of peri-implantitis was significantly more effective in reducing PD as compared with nonsurgical therapy. However, there are no long-term clinical trials in indexed literature that have compared nonsurgical with surgical interventions for peri-implantitis.
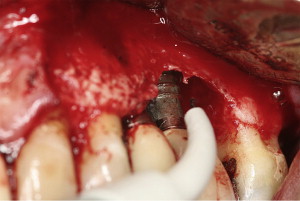
The surgical treatment approach is performed when an acute infection has resolved and appropriate oral hygiene of the patient is achieved. Guided bone regeneration (GBR) is a surgical approach in which a particulate graft material (such as allograft, xenograft, or alloplast) is directly placed into the osseous defect and covered with a barrier membrane (such as collagen membrane or expanded polytetrafluoroethylene [ePtFE] membrane). Schou and colleagues investigated the efficacy of GBR by using autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis in cynomolgus monkeys. In these studies, experimental peri-implantitis was induced in the jaws of monkeys after 3 months of the placement of Ti plasma-sprayed implants. Plaque-control protocols were executed and implants were divided into 4 different surgical treatment groups that comprised: (1) placement of autogenous bone grafts over the defect and covered by membranes, (2) covering the defect with autogenous bone grafts alone, (3) covering the defect with membrane alone, or (4) access flap procedure as a control group. Euthanasia was performed after 6 months, and jaw segments were assessed by quantitative digital subtraction radiography. The results showed that bone levels corresponding to levels that existed before induction of peri-implantitis were achieved in cases in which autogenous bone graft particles were placed over the defect and covered with membrane as compared with other groups.
The use of growth factors as adjuncts to conventional GBR protocols also has been reported to enhance osseointegration and promote new bone formation around induced peri-implant defects as compared with when GBR is performed without adjunct growth factor therapy. Although studies have reported that surgical management of peri-implant diseases yields promising results, long-term controlled studies are needed to validate the efficacy of these surgical treatments.
Lasers in the Treatment of Peri-implant Diseases
Some studies have recommended the use of lasers as an adjunct to the conventional nonsurgical treatment of peri-implantitis. It has been shown that carbon dioxide (CO 2 ) and 980-nm diode lasers do not affect implant surfaces during irradiation of implant surfaces as compared with other lasers, such as Neodymium-doped Yttrium-Aluminum-Garnet (Nd:YAG) and Erbium:YAG laser. In a case-series involving 15 patients, Romanos and Nentwig investigated the efficacy of CO 2 laser in the decontamination of failing implants. Clinical and radiologic parameters were evaluated at baseline and approximately after 2 years. The results showed almost complete bone fill in the peri-implant defects and the study concluded that decontamination of the implant surfaces with the CO 2 laser is an effective treatment for the management of peri-implantitis. Roncati and colleagues compared the effect of using a 810-nm diode laser as adjunct to conventional nonsurgical and nonsurgical debridement alone (control) in the treatment of peri-implantitis. The 5-year follow-up outcomes showed nonsurgical debridement with adjunctive 810-nm diode laser therapy is a more effective treatment strategy for the management of peri-implantitis as compared with nonsurgical debridement alone. Likewise, results from another study reported osteoblastic attachment to implant surfaces and bone formation is higher in irradiated as compared with nonirradiated implant surfaces.
Recently, a new technique termed laser-assisted peri-implantitis protocol (LAPIP) is under investigation. The LAPIP technique is an implant-specific modification of the laser-assisted new attachment protocol (LANAP). LAPIP and LANAP protocols use a laser-ablation step to eradicate inflamed sulcular tissues (using Nd:YAG laser) and decontaminate the implant/root surface followed by nonsurgical periodontal therapy (scaling and root planing). The LAPIP works on the concept of creation of a blood clot, which allows the defect area to heal apico-coronally by preventing the down-growth of gingival epithelium. However, there are no controlled trials in indexed literature regarding the efficacy of LAPIP for the management of peri-implantitis.
Photodynamic Therapy in the Treatment of Peri-implant Diseases
The concept of photodynamic therapy (PDT) is based on the application of light (generally red light with wavelength ranging between 630 and 700 nm) on a chemical dye (photosensitizer [PS]), which leads to the production of singlet oxygen molecules under aerobic conditions ( Fig. 8 ). These molecules have been reported to cause oxidative damage to the target cells, such as microbial and tumor cells. PDT has been shown to be a noninvasive and practical and feasible treatment option for periodontitis and cancer. Although clinical and experimental studies have investigated the efficacy of PDT in the management of peri-implantitis, there seems to be a lack of consensus among clinicians and researchers in nominating PDT as a treatment of choice for peri-implantitis. The factors associated with this discrepancy include variation in the study design, duration/frequency of treatment, selection criteria of control group and the laser-related parameter, such as type of laser PS. Thus, further long-term randomized controlled trials are needed to assess the role of PDT in the treatment of peri-implantitis.