Since the inception of the specialty of oral and maxillofacial surgery, the delivery of anesthesia has been the cornerstone of our practice. The control of pain and anxiety is a valuable service to the public, especially for the pediatric population. The majority of pediatric patients exhibit fear and anxiety when coming in for surgical treatment, and providing care for the pediatric patient can be a challenging but rewarding experience. When behavioral management techniques fail or when they are not practical, pharmacologic intervention will be necessary. The goals of pediatric anesthesia are to provide efficient, safe, reversible, and profound anesthesia, sedation, or analgesia to allow safe and effective completion of the surgical procedure.
Children are not simply small adults. Children have unique anatomic and physiologic differences that provide special challenges during anesthetic management. Maintaining a patent airway can be problematic as a result of large tonsils and a pliable funnel shaped trachea to name a few. Airway loss with subsequent hypoxia and resultant cardiovascular compromise account for the majority of poor outcomes during pediatric anesthesia.
Preanesthetic assessment for children should have areas of focus different from that of adults. A complete review of systems must include screening for endocrine disorders, congenital cardiac defects, hematologic disorders, and any familial history of malignant hyperthermia. Children tend to have more frequent upper respiratory tract infections and a higher incidence of asthma, and thorough investigation is critical.
Safety in the practice of state-of-the-art anesthesia is of the utmost importance to all oral and maxillofacial surgeons. Conditions contributing to major morbidity from office anesthetic procedures include unfamiliarity with pediatric anesthesia. One study concluded that the judgment and skill of the provider was one of the prime determinants related to anesthetic mortality. Constant review of the literature, having a thorough knowledge of current advances in technology and techniques, and ensuring maintenance of pediatric anesthesia skills are essential to providing the highest quality of care for our young patients.
This chapter discusses pediatric anatomy and physiology, preanesthetic work-up, and equipment and monitoring for the safe and effective delivery of anesthesia. Current anesthetic drugs and techniques are reviewed along with the more common complications and their prevention and management.
▪
PEDIATRIC ANATOMIC/PHYSIOLOGIC/PHARMACOLOGIC DIFFERENCES
The successful management of pediatric patients undergoing anesthesia requires a thorough understanding of the anatomic and physiologic differences between pediatric patients and adults. These differences lead to many modifications in the delivery of anesthesia from the preanesthetic evaluation to the actual delivery of pharmacologic agents.
CARDIOVASCULAR SYSTEM
The cardiovascular system undergoes significant changes at birth and continues to change throughout childhood. Transitional circulation describes the changes that take place as the fetus adjusts to the circulatory changes after birth. The end product of these changes includes the closure of the foramen ovale, closure of the ductus arteriosus, and closure of the ductus venosus, the combination of which causes the fetal circulation to become an adult-type circulation. These shunts do not close definitively immediately after birth; as a result, certain clinical conditions may contribute to the persistence of fetal circulation or to the reappearance of fetal shunts under stress. Factors, such as hypoxia, hypercarbia, acidosis, infection, hypothermia, and anesthesia-induced changes in peripheral vascular tone, act as a stimulus for maintaining high pulmonary vascular resistance and a return to fetal circulation. Blood may be shunted past the lungs through the patent foramen ovale, and a rapid downhill spiral may occur and result in severe hypoxemia. Furthermore, after the neonatal period, it is estimated that 50% of children younger than 5 years of age and 28% of adults will demonstrate probe patency of the foramen ovale, indicating the need for careful elimination of all air bubbles from an intravenous (IV) line in a pediatric patient.
Cardiac Output and Blood Pressure
Cardiac output in pediatric patients is higher than in adults and is necessary to meet the higher demands for metabolic oxygen consumption. Cardiac output is defined as stroke volume multiplied by heart rate. The myocardial structure of the pediatric heart is significantly less developed than in adults. In particular the volume of cellular mass devoted to contractility is less in the pediatric heart resulting in a noncompliant and poorly developed left ventricle that has minimal ability to alter stroke volume. Therefore without the ability to alter stroke volume, cardiac output in a pediatric heart is dependant on heart rate.
Mean arterial blood pressure is proportionate to cardiac output multiplied by systemic vascular resistance. The pediatric cardiovascular system has little ability to alter systemic vascular resistance; it is less able to respond to hypovolemia with vasoconstriction and is very sensitive to volume overloading as well with little ability to compensate. Therefore blood pressure in the pediatric patient is largely dependant on cardiac output. Bradycardia is the most lethal arrhythmia in pediatric patients leading to large decreases in cardiac output and a resultant decrease in blood pressure. Perhaps the most frequent cause of bradycardia in pediatric patients is hypoxemia. Other frequent causes include anesthetic overdose or activation of the parasympathetic nervous system ( Table 7-1 ).
| Age in Years | 1 | 3 | 6 | 12 | Adult |
|---|---|---|---|---|---|
| Respiration rate/min | 25-35 | 20-30 | 18-25 | 15-22 | 8-15 |
| Heart rate/min | 100-140 | 80-125 | 80-120 | 75-110 | 60-80 |
| Blood pressure mm Hg | 90/60 | 100/60 | 110/60 | 110/65 | 125/80 |
Neural Innervation
In pediatric patients, the sympathetic nervous system is not fully developed, giving the parasympathetic nervous system greater control and increased tone. A severe and sudden bradycardia can often be the result of this parasympathetic hypertonia or “increased vagal tone.” The clinician should always be prepared to treat a vagal induced bradycardia because it can happen in people of all ages; however, it is important to note that the incidence is inversely proportional to age with a sharp decline in incidence for children over the age of 3 ( Table 7-2 ).
| 0-1 Yr | 1-2 Yr | 2-3 Yr | 3-4 Yr | |
|---|---|---|---|---|
| Total anesthetics | 4645 | 1932 | 774 | 628 |
| With bradycardia | 59 | 19 | 5 | 1 |
| % Bradycardia | 1.27 | .98 | .65 | .16 |
RESPIRATORY SYSTEM
During the first years of life, the respiratory system undergoes significant changes. Immaturity of the respiratory system in young children (under age 2) makes anesthesia particularly dangerous and should only be attempted by providers adept in pediatric anesthesia. This section will discuss the changes that occur in the development of the respiratory system, which will be divided into the upper airways, the chest wall, and the lungs.
Upper Airways
Differences in airway anatomy between pediatric and adult airways make airway management and intubation difficult in pediatric patients. The upper airway of infants differs in a number of ways making pediatric patients more prone to obstruction than adults. First the infant’s tongue is large in relation to the oral cavity. Second the laryngeal cartilage is located higher in the neck. Third the epiglottis is large and can cover the soft palate, which makes the airway more prone to obstruction and encourages nasal breathing, often referred to as “obligatory” nasal breathing. Most infants can convert to oral breathing if the obstruction lasts more than 15 seconds, and almost all infants can easily convert to oral breathing by 5 months of age. Young children also have proportionately larger tonsils than adults, with tonsils and adenoids reaching their largest size between ages 4 to 10.
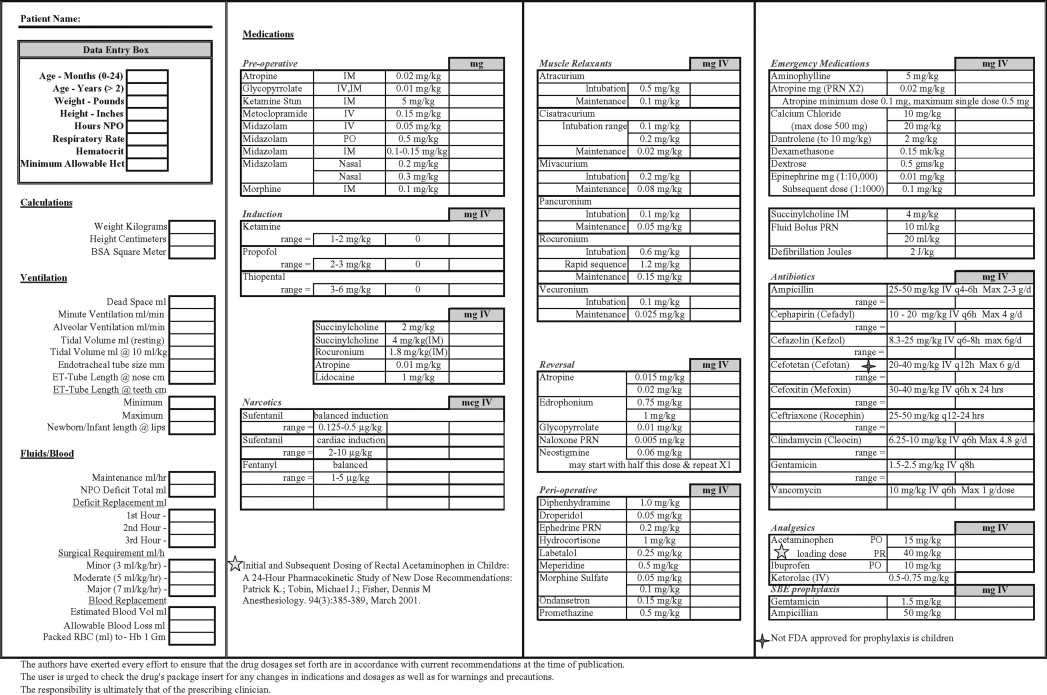
Endotracheal intubation is also influenced by the upper airway anatomy of the pediatric patient. The pediatric larynx is funnel shaped and is narrowest at the cricoid cartilage, whereas the narrowest part of the adult airway is the glottic opening ( Figure 7-1 ).
For this reason, endotracheal tubes that pass through the vocal cords of an adult will sit passively in the trachea. However, in pediatric patients, an endotracheal tube that passes through the vocal cords may be tight just below the cords in the area of the cricoid cartilage. Any endotracheal tube, cuffed or uncuffed, that is placed in a pediatric patient must be checked to ensure air will “leak” around the tube at no more than 25 cm H 2 O. At pressures greater than 25 cm of H 2 O. The mucosal capillaries may become damaged leading to subglottic inflammation, a dangerous phenomenon leading to further narrowing of an already narrow airway. The small diameter of the airways and further narrowing profoundly increases resistance to airflow because resistance is inversely proportional to the radius raised to the fourth power.
Over the first 2 years of life, the epiglottis, larynx, and hyoid bone move downward. The facial skeleton grows vertically, and the mandible lengthens from front to back. This growth continues until the age of 10 to 12 when the pediatric airway fully develops into an adult airway.
Another difference between the pediatric and adult upper airway is that the pediatric trachea is more compliant. The negative pressures generated during inspiration tend to collapse the trachea. The most compliant region of the upper airway is the pharynx, which makes this region most susceptible to collapse. The pharynx is usually protected from collapsing by the tone of the upper airway muscles, which also contract during inspiration. However, small upper airways have a higher resistance to flow and may necessitate greater negative pressures to produce sufficient inspiratory flow. This results in a greater tendency for the pediatric upper airway to collapse.
Chest Wall
There are several properties of the chest wall and associated muscles of respiration that diminish the efficacy of ventilation in the pediatric population.
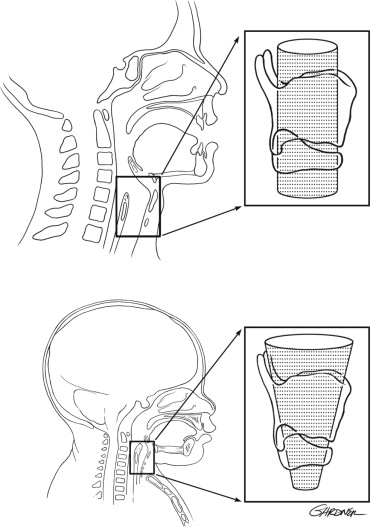
Pediatric ribs are very pliable because they are composed mainly of cartilage. As a result, the chest wall of an infant is very compliant and cannot easily maintain negative intrathoracic pressure, resulting in a decreased mechanical efficiency and less ventilatory reserve. In the infant, the compliance of the chest wall is almost three times that of the lung. By the age of 3, the ribs mineralize and become stiffer, making the chest wall and lung compliance similar. The efficacy of ventilation in the pediatric patient is further diminished by the anatomy and composition of the respiratory muscles, which bear the load of respiration. In an infant, the diaphragm inserts in an almost horizontal fashion, compared with the oblique insertion in an adult ( Figure 7-2 ).
As a result, there is decreased contraction efficiency in infants; the flat diaphragm has a shorter downward course with less rib cage expansion. In addition to having a decreased inspiratory capacity, the composition of the intercostal muscles and the diaphragm allow little room to increase ventilation in the face of increased work of breathing. The diaphragm is made up of a mixture of fatigue-resistant and fatiguesusceptible muscle fibers. The number of fatigue-resistant fibers in the diaphragm at birth is approximately ½ of the adult level. By 2 years of age, the diaphragm has matured to have approximately the same number of fatigue-resistant fibers as an adult. This decreased inspiratory capacity coupled with less fatigue-resistant fibers places the pediatric patient at a higher risk to develop ventilatory fatigue quickly after small changes in respiratory load.
Lungs
There are several properties of the lungs that influence anesthesia delivery to the pediatric patient. Many anatomic differences exist between the pediatric and adult lung.
Respiration is not possible until both the pulmonary airways and vascular system have matured enough to allow gas exchange. Marginal gas exchange capability is possible by approximately the 24th week of gestation, which is a critical step in allowing postnatal survival. Alveoli form both before and after birth, and initial studies suggested that postnatal alveolar multiplication was slow after the age of 4 and ended by the age of 8. However, more recent studies with larger groups of cases have shown that the great bulk of alveoli are present by the age of 2, and limited or no alveolar multiplication occurs thereafter. Following the end of alveolar multiplication, lung development is considered complete, and the lung enters a period of normal growth until adulthood.
In the newborn, the partial pressure of oxygen in arterial blood (PaO 2 ) is approximately 70 mm Hg, versus 97 mm Hg in the adult. In theory the low PaO 2 values may be due to ventilation-perfusion mismatch and a short capillary transit time that does not allow proper diffusion between the alveolar-capillary interface. The PaO 2 rises rapidly during the first 3 years of age with children aged 1 to 3 having an average PaO 2 of 83 mm Hg. The PaO 2 then slowly increases up to the age of 8 years. Thereafter, PaO 2 values remain stable and similar to those seen in adults. The fairly low PaO 2 values in infants and young children are close to the steep part of the oxygen-hemoglobin dissociation curve. Any further decrease in PaO 2 can induce severe oxygen desaturation.
The physiology of the pediatric lung is affected by the limited number of alveoli and compliant rib cage. Functional residual capacity (FRC) is the volume of gas remaining in the lung at the end of a normal tidal expiration. It represents the balance point between the inward elastic recoil of the lungs and the outward elastic recoil of the chest wall. In infants the chest wall is very compliant making the outward elastic recoil very small. Conversely the lung is stiff making inward elastic recoil high, possibly as a result of the small and limited number of alveoli. As a result of this balance, the FRC of a pediatric patient is lower than that of an adult, promoting low residual lung volumes at expiration. During anesthesia FRC represents oxygen stores during periods of apnea. The closing capacity is the volume of air that is needed to keep the airways open. If FRC falls below the closing capacity, airway closure occurs leading to a shunt. The closing capacity is greater in the pediatric patient. A decreased FRC coupled with an increased closing capacity predisposes the pediatric patient to shunting, or perfusing areas of the lung that are receiving no ventilation. This requires more energy on top of an already higher metabolic demand and oxygen consumption rate. Clinically, this correlates to rapid oxygen desaturation during periods of apnea (e.g., during intubation or airway obstruction). In one model describing the rate of oxyhemoglobin desaturation during apnea, a child took 41 seconds to desaturate to 85%, whereas an adult took 84 seconds to desaturate to 85% ( Table 7-3 ).
| Newborn | 3 Yr | 5 Yr | 12 Yr | Adult | |
|---|---|---|---|---|---|
| Tidal volume | 21 | 112 | 270 | 480 | 575 |
| Minimum ventilation | 1.05 | 2.46 | 5.5 | 6.2 | 6.4 |
| Vital capacity | 120 | 870 | 1160 | 3100 | 4000 |
| FRC | 80 | 490 | 680 | 1970 | 3000 |
KIDNEYS
Pediatric patients have decreased renal function under the age of 2. Multiple renal differences are seen early in life including decreased glomerular filtration rate, impaired sodium retention, and impaired glucose excretion. At birth glomerular filtration rate is approximately 15% to 30% of normal adult values. As a result, renal clearance of drugs is diminished. In addition, neonates are “obligate sodium losers” because they resorb less sodium in response to aldosterone than adult kidneys. Finally, glucose excretion is impaired in neonates. This is intended to offset the impaired glycogen stores present in neonates and tendency toward hypoglycemia. Meticulous drug, fluid, and glucose regulation is important in early life before renal function maturation. Functional maturation may be delayed in premature infants; however, by the age of 2, functional maturation should be complete.
LIVER
At birth the liver is functionally immature, with minimal glycogen stores that may lead to hypoglycemia. This is counterbalanced by decreased renal excretion of glucose as previously discussed. An immature liver is also reflected in immature liver enzyme systems. The cytochrome P-450 system, responsible for phase 1 drug metabolism of many lipophilic compounds, reaches approximately 50% of adult values at birth suggesting decreased capacity for drugs metabolized by this system. Phase II reactions involving drug conjugation, which facilitates renal excretion, is also impaired. These immature reactions alter drug metabolism and lead to long drug half-lives. By age 1, these reactions achieve adult activity.
THERMOREGULATION
Pediatric patients have a greater surface area to body weight ratio, or a larger surface area per kilogram than adults. They also have less muscle mass and less fat content than adults; as a result, pediatric patients quickly lose body heat to the environment. This makes pediatric patients more prone to hypothermia, which is associated with cardiac instability, respiratory depression, increased pulmonary vascular resistance, altered drug responses, and slower awakening from anesthesia. With a limited ability to shiver, infants respond to the stresses of a cold environment by increasing release of norepinephrine that enhances brown fat metabolism, a process called nonshivering thermogenesis. Environmental temperature is closely linked to the metabolic rates of infants, providing important sensory input. Under resting conditions, ambient temperature is the most important determinant of metabolic rate. An infant’s metabolic rate is the lowest when environmental temperatures are within a neutral range. Cold stress will lead to increased metabolic rate of brown fat via nonshivering thermogenesis, increased oxygen consumption, hypoxemia, and metabolic acidosis.
DEVELOPMENTAL PHARMACOLOGY
Pediatric patients have a dramatically different response than adults to medications. Their response is altered by body composition, distribution of cardiac output, protein binding, and functional maturity of the liver and kidneys. As a result, drugs that have a very predictable response in adults may have a very unpredictable response in children.
The body composition of a person changes with age. The total body water in a term infant accounts for approximately 73% of body weight versus 60% for an adult. As the child grows, total body water decreases as fat and muscle content increase. The implications of a drastically different body composition are twofold. First, drugs that are water soluble have a larger volume of water in which to distribute, which leads to initially larger doses to achieve the desired effect. Second, with less fat and muscle content, drugs that depend on redistribution into fat or muscle will remain intravascularly longer, leading to a longer clinical effect.
Distribution of cardiac output is different in pediatric patients, which results in unpredictable drug behavior. Pediatric patients have a higher cardiac output to vessel-rich groups (brain), which leads to a rapid onset of drugs. Pediatric patients also have decreased plasma levels of albumin leading to less protein binding and subsequently greater levels of free/active drug in the serum. Lastly, immature hepatic and renal function lead to long drug half-lives and decreased excretion.
As children grow, their body composition, distribution of cardiac output, protein binding, and functional maturity of the liver and kidneys all change to further affect drug behavior. The body composition of a child over the age of 2 is similar to that of an adult; they have normal adult values for protein, and the liver and kidney have functional maturity. Of particular importance is that a greater proportion of cardiac output is directed toward the already matured liver and kidneys, giving many medications a shorter half-life. As the child approaches adulthood, the half-life of many drugs lengthens to adult values. Overall most medications will have a prolonged elimination half-life in infants, a shortened half-life in children aged 2 to the early teen years, and a lengthening of half-life in those approaching adulthood.
▪
PREANESTHETIC ASSESSMENT
The ultimate goal of any preanesthetic assessment is to establish a treatment plan for the patient. Many medical problems discovered on the preanesthetic visit will prompt a change in patient management. One study found that the preanesthetic evaluation leads to changes in the treatment plan for more than 15% of American Society of Anesthesiologists (ASA) class 1 and 2 patients. Although a similar study has not been conducted in pediatric patients alone, the significance of the study is understood. A focused history and physical examination with special attention to those medical conditions that will prompt a change in treatment is necessary. Whereas some medical conditions that require a change in patient management will be obvious and would not escape the attention of most parents (i.e., insulin-dependant diabetes mellitus, congenital cardiac defects), others are quite obscure and may only be brought to light with focused questions. Specific questions to ask should focus around gestational age, recent upper respiratory infection (URI), asthma, gastroesophageal reflux, and history of bleeding problems or hematologic disorders. Premature infants can have multiple medical problems usually caused by immaturity of major organ systems or intrauterine asphyxia. Pulmonary complications of prematurity, including bronchopulmonary dysplasia, are particularly important to ask about because normal respiratory function may be delayed for many years, even up until age 6. It is also important to ask about history of previous anesthesia and family history of musculoskeletal diseases or malignant hyperthermia.
The physical examination should include the child’s general appearance, alertness, color, and activity level. Motor and verbal skills are also noted. Vital signs including height and weight are recorded. The physical exam should also focus on the cardiovascular, pulmonary, airway, and any other systems as indicated from the patient history. The presence of a heart murmur in a healthy, asymptomatic child is unlikely to indicate any significant cardiac pathologic condition. Innocent murmurs are usually soft systolic ejection murmurs that are best heard along the upper left or lower left sternal border without significant radiation and may occur in more than 30% of normal children. Endocarditis prophylaxis is required for children with congenital heart defects. If any question exists in regard to the significance of a heart murmur, consultation with a pediatrician or pediatric cardiologist is recommended.
According to the American Association of Oral and Maxillofacial Surgeons clinical practice guidelines for anesthesia in outpatient facilities, most poor outcomes in pediatric anesthesia are related to loss of the airway. The airway exam is extremely important in the physical examination of any patient undergoing anesthesia and especially in the pediatric patient with little respiratory reserve.
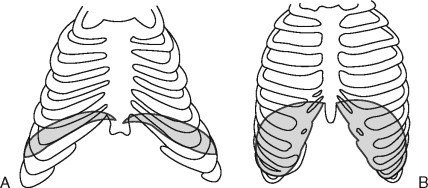
The airway exam should focus around three main characteristics that will help to detect a difficult airway. The first characteristic assesses the size of the tongue in relation to the oral cavity. The patient opens their mouth as widely as possible and protrudes their tongue as far as possible. The airway can then be classified according to what pharyngeal structures can be seen, also known as the “Mallampati” classification ( Figure 7-3 ).
This classification is important because the ability to see vocal cords on direct laryngoscopy is almost always good in class 1 patients and almost always poor in class 4 patients. Additional factors that can complicate airway management include large tonsils and loose teeth. The second characteristic assesses the atlanto-occipital joint extension. If the neck can be flexed to approximately 25° to 35°, the atlanto-occipital joint is extended, and the oral, pharyngeal, and laryngeal structures are nearly aligned, allowing for easier laryngoscopy. It should be noted that children with Down syndrome may have atlanto-occipital joint instability, and extension of the neck during intubation may lead to cervical dislocation and spinal cord trauma. Appropriate clinical assessment including extension and flexion lateral neck films may be necessary to detect instability before anesthesia for these patients. The third characteristic in evaluation of the airway is measuring the mandibular space, also known as the thyromental distance. This is the space anterior to the larynx and is an indication for how easily the laryngeal axis will align with the pharyngeal axis during direct laryngoscopy. This value is expressed by the number of “fingerbreadths,” with more “fingerbreadths” being indicative of easier laryngoscopy. Neonates should have at least one “fingerbreadth” and adolescents at least three to ease in direct laryngoscopy.
PREOPERATIVE FASTING
Pediatric patients are more prone to dehydration than adults, as a result their preoperative fasting guidelines have been less strict. Additionally, asking a pediatric patient to fast for an extended period of time will lead to increased irritability of the patient. Conversely the incidence of aspiration is three times higher in pediatric patients than adults (9 per 10,000 versus 3 per 10,000), indicating the need for careful attention to the matter. Fortunately, if aspiration does occur, serious respiratory morbidity is rare in the immediate postoperative period in pediatric patients. In 1999 the ASA appointed a task force to establish practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration in healthy patients undergoing elective procedures ( Table 7-4 ).
| Ingested Material | Minimum Fasting Period (Hr) |
|---|---|
| Clear liquids | 2 |
| Breast milk | 4 |
| Infant formula | 6 |
| Nonhuman milk | 6 |
| Light meal | 6 |
The ASA points out that with clear liquids there is no difference in the gastric volume of children who fasted 2 to 4 hours versus more than 4 hours. Examples of clear liquids include water, fruit juices without pulp, and carbonated beverages. The ASA also points out that nonhuman milk is similar to solids in gastric emptying time, and therefore the amount ingested must be considered when determining an appropriate fasting time. Finally, ASA notes that a “light meal” typically consists of toast and clear liquids. Meals that include fried, fatty foods, or meat may prolong gastric emptying leading to a fasting recommendation of 8 or more hours before elective procedures. Whereas some published evidence supports the routine use of pharmacologic agents to reduce the risk of pulmonary aspiration, the ASA states that routine use of pharmacologic agents to reduce the risk of pulmonary aspiration in patients who have no apparent increased risk for aspiration is not recommended.
UPPER RESPIRATORY INFECTION
During the preanesthetic evaluation, it is important to ask if the child has had any recent colds that may indicate a recent upper respiratory infection (URI). If the child has in fact had a recent cold, it is important to differentiate between a URI and simple rhinitis. Clear rhinorrhea is usually not a concern, and anesthesia is not contraindicated. A URI is usually associated with fever, mucopurulent green or yellow discharge, and a productive cough. Children with URIs have irritable airways and are at an increased risk for laryngospasm, breath holding, postintubation croup, atelectasis, pneumonia, and episodes of desaturation. Furthermore airway hyperreactivity may last for up to 6 weeks after a URI, and the incidence of complications in children recovering from a URI is almost the same as children who have active URIs. The presence of URI in a child is particularly important because increased airway edema and inflammation in an already narrow airway will cause profound increases in resistance to airflow. Traditional guidelines for treatment of patients with URIs suggest that the procedure be delayed for 4 to 6 weeks after cessation of the URI to decrease the risk to the patient.
ASTHMA
Asthma is one of the most common chronic conditions in the United States, with the prevalence being highest for people under the age of 18. Moreover, the prevalence among children aged 5 to 14 has increased 74% from 1980 to 1994 and 160% among children under the age of 4 from 1980 to 1994. Given the degree of prevalence among the pediatric population it is likely that oral and maxillofacial surgeons will treat pediatric patients with asthma. Treating pediatric patients with moderate to severe asthma can be particularly dangerous because the overall mortality rates of asthma are highest between the ages of 5 to 14. Moreover, acute and fatal bronchospasm can occur during anesthesia and endotracheal intubation in children with asthma. Whenever possible endotracheal intubation should be avoided in these patients.
In patients with asthma, elective cases should not be undertaken unless their asthma is well controlled. During preoperative assessment of the asthmatic patient, it is important that the disease is assessed by the cause, frequency and severity of attacks, hospital and intensive care admissions, and by drug history. A thorough examination is required, which may reveal expiratory wheezes, use of accessory muscles, or an overdistended chest. If a child is actively wheezing, elective surgery should be canceled. A child should be free of wheezing for at least 1 week before surgery. Attempts to control wheezing with an increase in β-agonists or the addition of steroids are indicated preoperatively. Preoperative steroids may be needed for children with asthma who continue to wheeze despite an increase in β-agonists or as a stress dose for children who have received steroids over the last year to control their asthma. The addition of prednisone, 1 mg/kg given 24 and 12 hours before surgery will considerably decrease airway reactivity. If preoperative wheezing cannot be controlled with conservative therapy as an outpatient, admission for more aggressive therapy and consultation with a pediatrician is indicated.
▪
PREANESTHETIC ASSESSMENT
The ultimate goal of any preanesthetic assessment is to establish a treatment plan for the patient. Many medical problems discovered on the preanesthetic visit will prompt a change in patient management. One study found that the preanesthetic evaluation leads to changes in the treatment plan for more than 15% of American Society of Anesthesiologists (ASA) class 1 and 2 patients. Although a similar study has not been conducted in pediatric patients alone, the significance of the study is understood. A focused history and physical examination with special attention to those medical conditions that will prompt a change in treatment is necessary. Whereas some medical conditions that require a change in patient management will be obvious and would not escape the attention of most parents (i.e., insulin-dependant diabetes mellitus, congenital cardiac defects), others are quite obscure and may only be brought to light with focused questions. Specific questions to ask should focus around gestational age, recent upper respiratory infection (URI), asthma, gastroesophageal reflux, and history of bleeding problems or hematologic disorders. Premature infants can have multiple medical problems usually caused by immaturity of major organ systems or intrauterine asphyxia. Pulmonary complications of prematurity, including bronchopulmonary dysplasia, are particularly important to ask about because normal respiratory function may be delayed for many years, even up until age 6. It is also important to ask about history of previous anesthesia and family history of musculoskeletal diseases or malignant hyperthermia.
The physical examination should include the child’s general appearance, alertness, color, and activity level. Motor and verbal skills are also noted. Vital signs including height and weight are recorded. The physical exam should also focus on the cardiovascular, pulmonary, airway, and any other systems as indicated from the patient history. The presence of a heart murmur in a healthy, asymptomatic child is unlikely to indicate any significant cardiac pathologic condition. Innocent murmurs are usually soft systolic ejection murmurs that are best heard along the upper left or lower left sternal border without significant radiation and may occur in more than 30% of normal children. Endocarditis prophylaxis is required for children with congenital heart defects. If any question exists in regard to the significance of a heart murmur, consultation with a pediatrician or pediatric cardiologist is recommended.
According to the American Association of Oral and Maxillofacial Surgeons clinical practice guidelines for anesthesia in outpatient facilities, most poor outcomes in pediatric anesthesia are related to loss of the airway. The airway exam is extremely important in the physical examination of any patient undergoing anesthesia and especially in the pediatric patient with little respiratory reserve.
The airway exam should focus around three main characteristics that will help to detect a difficult airway. The first characteristic assesses the size of the tongue in relation to the oral cavity. The patient opens their mouth as widely as possible and protrudes their tongue as far as possible. The airway can then be classified according to what pharyngeal structures can be seen, also known as the “Mallampati” classification ( Figure 7-3 ).
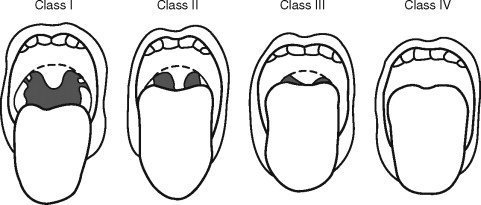
This classification is important because the ability to see vocal cords on direct laryngoscopy is almost always good in class 1 patients and almost always poor in class 4 patients. Additional factors that can complicate airway management include large tonsils and loose teeth. The second characteristic assesses the atlanto-occipital joint extension. If the neck can be flexed to approximately 25° to 35°, the atlanto-occipital joint is extended, and the oral, pharyngeal, and laryngeal structures are nearly aligned, allowing for easier laryngoscopy. It should be noted that children with Down syndrome may have atlanto-occipital joint instability, and extension of the neck during intubation may lead to cervical dislocation and spinal cord trauma. Appropriate clinical assessment including extension and flexion lateral neck films may be necessary to detect instability before anesthesia for these patients. The third characteristic in evaluation of the airway is measuring the mandibular space, also known as the thyromental distance. This is the space anterior to the larynx and is an indication for how easily the laryngeal axis will align with the pharyngeal axis during direct laryngoscopy. This value is expressed by the number of “fingerbreadths,” with more “fingerbreadths” being indicative of easier laryngoscopy. Neonates should have at least one “fingerbreadth” and adolescents at least three to ease in direct laryngoscopy.
PREOPERATIVE FASTING
Pediatric patients are more prone to dehydration than adults, as a result their preoperative fasting guidelines have been less strict. Additionally, asking a pediatric patient to fast for an extended period of time will lead to increased irritability of the patient. Conversely the incidence of aspiration is three times higher in pediatric patients than adults (9 per 10,000 versus 3 per 10,000), indicating the need for careful attention to the matter. Fortunately, if aspiration does occur, serious respiratory morbidity is rare in the immediate postoperative period in pediatric patients. In 1999 the ASA appointed a task force to establish practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration in healthy patients undergoing elective procedures ( Table 7-4 ).
| Ingested Material | Minimum Fasting Period (Hr) |
|---|---|
| Clear liquids | 2 |
| Breast milk | 4 |
| Infant formula | 6 |
| Nonhuman milk | 6 |
| Light meal | 6 |
The ASA points out that with clear liquids there is no difference in the gastric volume of children who fasted 2 to 4 hours versus more than 4 hours. Examples of clear liquids include water, fruit juices without pulp, and carbonated beverages. The ASA also points out that nonhuman milk is similar to solids in gastric emptying time, and therefore the amount ingested must be considered when determining an appropriate fasting time. Finally, ASA notes that a “light meal” typically consists of toast and clear liquids. Meals that include fried, fatty foods, or meat may prolong gastric emptying leading to a fasting recommendation of 8 or more hours before elective procedures. Whereas some published evidence supports the routine use of pharmacologic agents to reduce the risk of pulmonary aspiration, the ASA states that routine use of pharmacologic agents to reduce the risk of pulmonary aspiration in patients who have no apparent increased risk for aspiration is not recommended.
UPPER RESPIRATORY INFECTION
During the preanesthetic evaluation, it is important to ask if the child has had any recent colds that may indicate a recent upper respiratory infection (URI). If the child has in fact had a recent cold, it is important to differentiate between a URI and simple rhinitis. Clear rhinorrhea is usually not a concern, and anesthesia is not contraindicated. A URI is usually associated with fever, mucopurulent green or yellow discharge, and a productive cough. Children with URIs have irritable airways and are at an increased risk for laryngospasm, breath holding, postintubation croup, atelectasis, pneumonia, and episodes of desaturation. Furthermore airway hyperreactivity may last for up to 6 weeks after a URI, and the incidence of complications in children recovering from a URI is almost the same as children who have active URIs. The presence of URI in a child is particularly important because increased airway edema and inflammation in an already narrow airway will cause profound increases in resistance to airflow. Traditional guidelines for treatment of patients with URIs suggest that the procedure be delayed for 4 to 6 weeks after cessation of the URI to decrease the risk to the patient.
ASTHMA
Asthma is one of the most common chronic conditions in the United States, with the prevalence being highest for people under the age of 18. Moreover, the prevalence among children aged 5 to 14 has increased 74% from 1980 to 1994 and 160% among children under the age of 4 from 1980 to 1994. Given the degree of prevalence among the pediatric population it is likely that oral and maxillofacial surgeons will treat pediatric patients with asthma. Treating pediatric patients with moderate to severe asthma can be particularly dangerous because the overall mortality rates of asthma are highest between the ages of 5 to 14. Moreover, acute and fatal bronchospasm can occur during anesthesia and endotracheal intubation in children with asthma. Whenever possible endotracheal intubation should be avoided in these patients.
In patients with asthma, elective cases should not be undertaken unless their asthma is well controlled. During preoperative assessment of the asthmatic patient, it is important that the disease is assessed by the cause, frequency and severity of attacks, hospital and intensive care admissions, and by drug history. A thorough examination is required, which may reveal expiratory wheezes, use of accessory muscles, or an overdistended chest. If a child is actively wheezing, elective surgery should be canceled. A child should be free of wheezing for at least 1 week before surgery. Attempts to control wheezing with an increase in β-agonists or the addition of steroids are indicated preoperatively. Preoperative steroids may be needed for children with asthma who continue to wheeze despite an increase in β-agonists or as a stress dose for children who have received steroids over the last year to control their asthma. The addition of prednisone, 1 mg/kg given 24 and 12 hours before surgery will considerably decrease airway reactivity. If preoperative wheezing cannot be controlled with conservative therapy as an outpatient, admission for more aggressive therapy and consultation with a pediatrician is indicated.
▪
MONITORS
The ASA has set the standards for basic anesthetic monitoring for all anesthesia care. The goal of these standards is to encourage quality patient care while noting that it is but one component of care and that the monitors alone do not offer patient safety guarantees. Two basic standards rely on both provider vigilance and basic monitoring. Although the various monitors and alarms are a very important component of anesthesia care, they do not replace the anesthesia provider.
The first standard states that a qualified anesthesia provider must be present in the room through all stages of anesthesia care. The objective of this standard is based upon the need for a qualified anesthesia provider to monitor and address any of the possible rapid changes in patient status and to be present continuously. Although it is not currently necessary, it is highly recommended that providers administering anesthesia to children have current certification in Pediatric Advanced Life Support (PALS). The observation of levels of consciousness during anesthesia will reduce risks and most likely prevent many complications if adverse drug responses are detected in a timely manner.
The second standard states how oxygenation, ventilation, circulation, and temperature are to be continually monitored. Continually is further defined as “repeated regularly and frequently in steady rapid succession.”
Oxygen concentration is assessed in inspired gas and blood with pulse oximetry during any form of anesthesia delivery. Pulse oximetry will be accompanied by pitch pulse tone with audible, low threshold tone alarms. Adequate lighting will help with visual assessment of physical appearance to ascertain appropriate oxygenation of tissues. This is especially important in small children because of the rapid rate of desaturation and avoidance of hypoxic episodes.
Adequate ventilation should be evaluated by continual observance of qualitative clinical signs, such as chest expansion, reservoir breathing bag movements, and chest auscultation. Carbon dioxide detection is necessary during use of general anesthesia including verification of placement of an endotracheal tube or laryngeal mask airways (LMAs) and will be monitored continually unless undetectable as a result of nature of patient, procedure, or equipment. This will be done from initial placement of the airway device until removal. This measurement will be done with capnography, capnometry, or mass spectroscopy. Mechanical ventilation units must include an audible, disconnect alarm. All other forms of anesthesia care should also be evaluated by continual observation of clinical signs and/or detection of exhaled carbon dioxide, although it would be expected to be lower if using a nonclosed breathing circuit, such as a nasal canula, where the presence of exhaled carbon dioxide is the means of detection.
Circulation will be monitored by continuous electrocardiogram and heart rate display and arterial blood pressure display at minimum 5-minute intervals. Additional measures, such as pulse palpation or heart sound auscultation and intraarterial pressure tracings, could also be used for general anesthesia patients. Blood pressure cuffs should be appropriately fitted for each child to ensure correct measurements because a cuff that is too large will underestimate readings. Each patient should be sized with a blood pressure cuff that has a width 20% to 50% greater than the diameter of the patient’s extremity.
Every child receiving anesthesia shall have temperature monitoring when a clinically significant change in body temperature is intended, anticipated, or suspected.
ALTERNATIVE MONITORS
Adjuncts to standard monitors evaluate other clinical and physiologic processes. The use of a precordial stethoscope adds the ability to hear heart sounds and airway status easily. Bispectral analysis (BIS) is a noninvasive method to evaluate levels of anesthesia, and it is based on the principle that electroencephalogram (EEG) waveforms change with the level of alertness. The manufacturer has developed numeric value, known as the BIS index, based on an algorithm of EEG digital signals. The numeric value corresponds to level of sedation: 90 to 100 awake, 70 to 90 light to moderate sedation, 60 to 70 deep sedation, 40 to 60 general anesthesia, and less than 40 a deep hypnotic state. The BIS index has been shown to be closely associated to sedation scales, which would prevent possible oversedation.
Transcutaneous capnometry is being studied as an alternative method of detecting ventilatory malfunction and prevention of hypoxemia in patients in addition to pulse oximetry. Pulse oximetry is always accompanied with a delay, and a combination of the two provides the advantage of monitoring both alveolar ventilation (capnometry) and oxygen transport to the periphery (pulse oximetry) noninvasively. Stein et al. investigated two transcutaneous capnometers for clinical use, but do not currently recommend their use in clinical conditions in the current form because they provide only an approximate estimation of PaCO 2 .
ALARMS
Monitors and associated alarms may only alert the provider if they are activated. Block et al. reported that a majority of anesthesiologists routinely disable or mute audible alarms because of a number of false alarms. False alarms are a common source of disruption and distraction in the operating suite because they alert the anesthesia provider of known information that is already being controlled or monitored by the anesthesia provider. The Joint Commission on Accreditation of Healthcare Organizations and the Anesthesia Patient Safety Foundation (APSF) both recommend the use of audible alarms. Alarms have proven their importance by preventing and reducing the severity of anesthetic incidents, but sole reliance on monitors without clinical support may also lead to inappropriate interventions. Providers should become familiar with their equipment and determine the proper alarm settings based upon ASA guidance and clinical judgment.
▪
EQUIPMENT AND ROOM PREPARATIONS
The treatment of pediatric patients requires some adjustments to the setup in preparation for the procedure and presence of additional and often helpful elements in the operating arena. Some of these address emergency medications, equipment, fluid delivery access, and delivery. Initial preparations should be to change monitoring and anesthesia equipment to pediatric settings.
All pediatric operatories should have emergency equipment that is located in a well-known, easily accessible area. This equipment should be well maintained and inspected on a periodic basis. Appropriate algorithms and medicines to treat perioperative emergencies should be included in that location as well. A defibrillator with pediatric paddles along with emergency suction, auxiliary oxygen supplies, and alternative source of light should be available in case of loss of power. The oxygen source should be able to deliver under pressure for a minimum of 1 hour. At our institution, each pediatric patient has their information included in a worksheet that takes patient vital statistics and converts that information into appropriate equipment sizes, fluid replacement amounts, and doses of common perioperative medications. This form is then placed in the operative setting to be reviewed as needed ( Box 7-1 ).
Broselow Emergency Pediatric Tape provides a reference at each color bar on the tape to select equipment sizes to perform emergency resuscitation based on weight zone. The tape also shows precalculated medication dosages and infusion rates based upon each colored weight zone. Other commercial and institutional items are available to provide similar information.
An average setup of pediatric patient treatment begins with setup of room and equipment. Because infants and children have a greater surface area to body weight ratio causing greater body heat losses, temperature of the operating suite should be adjusted to between 80 °F to 90 °F. Most oral surgery procedures completed as outpatient procedures are relatively short, so clinical judgment should determine suitability of additional measures, such as warm fluid and heating blankets.
AIRWAY
Items needed to access and control the airway should be readily available; these include appropriately sized nasal cannulas, nasopharyngeal airways, oropharyngeal airway, endotracheal tubes, and LMAs ( Table 7-5 ).
| Mask Size | Patient Selection Guidelines | * Maximum Cuff Inflation Volume (Air) |
|---|---|---|
| 1 | Neonates/infants up to 5 kg | up to 4 mL |
| 1½ | Infants 5-10 kg | up to 7 mL |
| 2 | Infants/children 10-20 kg | up to 10 mL |
| 2½ | Children 20-30 kg | up to 14 mL |
| 3 | Children 30-50 kg | up to 20 mL |
| 4 | Adults 50-70 kg | up to 30 mL |
| 5 | Adults 70-100 kg | up to 40 mL |
| 6 | Large adults over 100 kg | up to 50 mL |
* These are maximum volumes that should never be exceeded. It is recommended the cuff be inflated to 60 cm H 2 O intracuff pressure.
Pocket masks and bag-mask-valve devices with positive pressure ventilation ability should be included. Inclusion of a system of emergent airway access, such as a cricothyrotomy kit, should be available in case situations arise where other methods have failed to establish a patent airway. Transtracheal catheter jet ventilation may be considered for support of oxygenation when a surgical airway is required for pediatric patients, but only in situations where oxygenation and ventilation cannot be provided any other way. Ravussin et al. used transtracheal jet ventilation during pediatric endoscopic laser treatment of laryngeal and subglottic stenosis. Adequate control of the airway and satisfactory gas exchange were obtained in all cases; however, they reported a severe life-threatening complication rate of 10.7% including pneumomediastinum and bilateral pneumothoraces.
A simple method to size an oropharyngeal airway is by placing the anterior portion at the commissure and the posterior portion to the angle of the mandible. Endotracheal tubes should also be available and sized appropriately. A common method for sizing an endotracheal tube for children over 2 years old is found in the formula:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses