Treatment of head and neck malignancies has evolved since the mid-1980s. Advances in surgical techniques, chemotherapeutic options, and radiation techniques have improved locoregional disease control and survival. Radiation therapy is used as the primary therapy for early stage malignancies, as adjuvant treatment following ablative surgery for advanced carcinomas and in patients with unresectable, locally advanced carcinomas, concurrent chemoradiotherapy has become the primary treatment modality.
In radiation therapy, the normal tissue tolerance is the dose-limiting factor for treatment of a malignancy. Mucosa, salivary gland tissue, skin, bone, and cartilage each have their own dose sensitivities and response to radiation-induced trauma. The side effects of radiation therapy are divided into acute and late toxicities, with each having an impact on the patient’s quality of life. Acute side effects include mucositis, xerostomia, pain, dermatitis, and dysgeusia, and late radiation-induced side effects include xerostomia, dysphagia, and skin fibrosis. Radiation-associated skeletal injuries differ in the adult and pediatric populations. In the pediatric population, the concerns center mostly on growth and development, whereas for the adult population it is the development of necrosis and pathologic fractures.
Osteoradionecrosis (ORN) was first described in 1922 by Regaud, and it remains a serious complication of radiation therapy. Patients afflicted with ORN in the head and neck region can experience pain, dysesthesia, trismus, orocutaneous fistula, and the late development of pathologic fractures. Utilizing data from pooled studies of head and neck radiated patients conducted between 1968 and 1992, Clayman reported a crude rate for ORN of 5.4%. More recently, Wahl estimated the incidence to be 3% when utilizing data from studies performed between 1997 and 2004. This current decline in ORN incidence appears to be multifactorial. The multidisciplinary care of head and neck cancer patients has provided for dental screening and preventive care to be instituted prior to the start of definitive therapy. This helps identify dental risk factors and addresses current and potential oral health problems for the radiation-therapy patient. The use of the more uniformly absorbed megavoltage radiation instead of kilovoltage therapy with its higher bone absorption, advances in 3D radiation planning, dose delivery monitoring, and computer-controlled delivery have resulted in 3D conformal radiation therapy and intensity-modulated radiation therapy (IMRT), which limit the radiation dose to surrounding normal tissue and associated treatment side effects.
Definition
Osteoradionecrosis has also been referred to as radiation osteitis, radio-osteonecrosis, radiation osteomyelitis, and postradiation osteonecrosis. There is currently no standardized clinical definition for ORN. Case series utilize different temporal and size criteria of chronic bone exposure in a radiated field when defining mandibular ORN. It has been defined as bone in the radiation field exposed for at least 2 months in the absence of local neoplastic disease and an area greater than 1 cm of exposed bone in a field of irradiation that has failed to show any evidence of healing for at least 6 months. The most commonly used definition is that of exposed, devitalized bone in a radiated field without healing for 3 months without any evidence of tumor recurrence.
Pathophysiology
Bones are resistant to high radiation doses as long as the overlying soft tissue remains intact. In 1938, Watson and Scarborough described three crucial factors in the development of ORN: exposure to radiotherapy above a critical dose, local injury, and the development of an infection. In 1960, Gowgiel used the Macacus rhesus monkey as an animal model to study the disease pathogenesis, total radiation dose needed for development of ORN, and the difference in disease incidence between the maxilla and mandible. His observations were that development of ORN was a result of direct damage to the osteocytes and that the vascular changes noted histologically played a role in disease progression. These vascular changes were found predominantly in the field of radiation and consisted of thickening of the arteriolar walls that resembled those seen in arteriosclerosis. The intima and media changes could be evident as early as ![]() weeks after completion of therapy, with complete occlusion observed within 5 to 7 weeks postradiation therapy. Gowgiel hypothesized that the difference in incidence of ORN between the maxilla and mandible centered on the vascular supply, with the maxilla having a rich vascular plexus when compared to the single inferior alveolar vessel for the mandible.
weeks after completion of therapy, with complete occlusion observed within 5 to 7 weeks postradiation therapy. Gowgiel hypothesized that the difference in incidence of ORN between the maxilla and mandible centered on the vascular supply, with the maxilla having a rich vascular plexus when compared to the single inferior alveolar vessel for the mandible.
In 1970, Meyer described the triad of radiation, trauma, and infection for the pathogenesis of ORN. Tissue injury provided the entry of oral bacteria to a compromised bed, which led to the development of an infection and necrosis. Marx, in 1983, examined several bone specimens to determine the pathogenesis of ORN. Bacteria were only identified superficially in the ORN specimens, and the conclusion was that organisms played a minor role in the pathophysiology of ORN and were not the causative agent but rather contaminants. Marx then characterized ORN as a wound healing complication and not an infectious process. In the radiated tissue, the hypovascular, hypoxic, and hypocellular environment would hinder a normal healing response to trauma and a chronic, nonhealing wound then develop. This became the theory of the pathophysiology of ORN for decades. It also served as the foundation for the use of hyperbaric oxygen therapy in the prevention and management of ORN because of its mechanism of action and its effect on radiated hard and soft tissue.
Molecular advances have allowed for a better understanding of the pathogenesis of osteoradionecrosis and the complexity of wound healing after radiation therapy. Osteoradionecrosis is now considered to be a late effect of radiation induced fibroatrophic process. Each fraction of radiation therapy represents a series of small tissue insults produced by a burst of free radicals. The process of inflammation and regeneration is altered by the influence of cytokines and growth factors on fibroblasts and the associated extracellular matrix deposition. Histopathologically, radiation-induced fibrosis is divided into three phases. In the prefibrotic phase , which is often asymptomatic, there are signs of chronic inflammation evident. The second phase is that of organized fibrosis , where there are areas of active fibrosis with a high concentration of myofibroblasts within a poorly organized matrix. These areas are adjacent to regions of aging fibroblasts in a poorly cellular, fibrotic, and dense sclerotic matrix. In the late fibroatrophic phase , retractile fibrosis and gradual loss of parenchymal cells takes place. The ability of radiated tissue to repair itself after an insult is then compromised by these cellular changes and can lead to the development of necrosis in susceptible individuals.
Pathophysiology
Bones are resistant to high radiation doses as long as the overlying soft tissue remains intact. In 1938, Watson and Scarborough described three crucial factors in the development of ORN: exposure to radiotherapy above a critical dose, local injury, and the development of an infection. In 1960, Gowgiel used the Macacus rhesus monkey as an animal model to study the disease pathogenesis, total radiation dose needed for development of ORN, and the difference in disease incidence between the maxilla and mandible. His observations were that development of ORN was a result of direct damage to the osteocytes and that the vascular changes noted histologically played a role in disease progression. These vascular changes were found predominantly in the field of radiation and consisted of thickening of the arteriolar walls that resembled those seen in arteriosclerosis. The intima and media changes could be evident as early as ![]() weeks after completion of therapy, with complete occlusion observed within 5 to 7 weeks postradiation therapy. Gowgiel hypothesized that the difference in incidence of ORN between the maxilla and mandible centered on the vascular supply, with the maxilla having a rich vascular plexus when compared to the single inferior alveolar vessel for the mandible.
weeks after completion of therapy, with complete occlusion observed within 5 to 7 weeks postradiation therapy. Gowgiel hypothesized that the difference in incidence of ORN between the maxilla and mandible centered on the vascular supply, with the maxilla having a rich vascular plexus when compared to the single inferior alveolar vessel for the mandible.
In 1970, Meyer described the triad of radiation, trauma, and infection for the pathogenesis of ORN. Tissue injury provided the entry of oral bacteria to a compromised bed, which led to the development of an infection and necrosis. Marx, in 1983, examined several bone specimens to determine the pathogenesis of ORN. Bacteria were only identified superficially in the ORN specimens, and the conclusion was that organisms played a minor role in the pathophysiology of ORN and were not the causative agent but rather contaminants. Marx then characterized ORN as a wound healing complication and not an infectious process. In the radiated tissue, the hypovascular, hypoxic, and hypocellular environment would hinder a normal healing response to trauma and a chronic, nonhealing wound then develop. This became the theory of the pathophysiology of ORN for decades. It also served as the foundation for the use of hyperbaric oxygen therapy in the prevention and management of ORN because of its mechanism of action and its effect on radiated hard and soft tissue.
Molecular advances have allowed for a better understanding of the pathogenesis of osteoradionecrosis and the complexity of wound healing after radiation therapy. Osteoradionecrosis is now considered to be a late effect of radiation induced fibroatrophic process. Each fraction of radiation therapy represents a series of small tissue insults produced by a burst of free radicals. The process of inflammation and regeneration is altered by the influence of cytokines and growth factors on fibroblasts and the associated extracellular matrix deposition. Histopathologically, radiation-induced fibrosis is divided into three phases. In the prefibrotic phase , which is often asymptomatic, there are signs of chronic inflammation evident. The second phase is that of organized fibrosis , where there are areas of active fibrosis with a high concentration of myofibroblasts within a poorly organized matrix. These areas are adjacent to regions of aging fibroblasts in a poorly cellular, fibrotic, and dense sclerotic matrix. In the late fibroatrophic phase , retractile fibrosis and gradual loss of parenchymal cells takes place. The ability of radiated tissue to repair itself after an insult is then compromised by these cellular changes and can lead to the development of necrosis in susceptible individuals.
Risk Factors
Multiple risk factors are associated with the development of mandibular ORN. These factors can be divided into treatment-dependent factors (radiation therapy factors and tumor-related factors) and patient-dependent factors. Among the radiation therapy factors, the type of radiation, its total dose, fractionation, and field have all been correlated with the development of ORN. The higher the total radiation dose, the higher the risk of ORN because of the tissue trauma that is sustained. Although a radiation dose of less than 60 Gy is associated with less risk of ORN, it is still not a negligible risk. In hyperfractionated radiation therapy, the total dose of radiation is divided into small doses and treatments are given more than once a day. This allows for total dose escalation to increase the rate of tumor control while limiting an increase in late complications. Conflicting data exist regarding the relationship of dose fractionation and the risk of ORN. A randomized, multicenter trial comparing continuous, hyperfractionated, accelerated radiotherapy (CHART) to conventional radiotherapy in 918 head and neck cancer patients found a 0.4% overall incidence of ORN in the CHART group compared to 1.4% for conventional radiotherapy. Niewald et al. showed in a retrospective analysis of 168 oral cavity carcinoma patients that ORN occurred in 8.6% of the patients treated conventionally when compared to 22.9% in those treated with hyperfractionation.
Tumors close to the mandible and those located in the tonsillar and retromolar trigone region appear to carry a higher risk of ORN. The treatment of these sites with radiation therapy places the posterior mandible where the buccal cortex appears to be more susceptible to the development of ORN in the treatment field. Mandibular surgery is, at times, required because of the extent of the tumor invasion or because its location calls for a wider access for proper oncologic surgery. The need for a mandibulotomy or mandibulectomy has also been correlated with a higher risk of ORN.
Patient age, dental status, oral hygiene, and use of tobacco have all been identified as risk factors for the development of ORN. In head and neck cancer patients, smoking cessation becomes an integral part of posttreatment surveillance, as it is not only associated with higher incidence of locoregional failure and development of a second primary tumor but also with osteoradionecrosis. A longitudinal study of the oral health of head and neck cancer patients found that patients who developed ORN experienced worsening of their periodontal status after completion of radiation therapy. The presence of greater than 60% alveolar bone loss, high dental plaque score, and pocket depths greater than 5 mm showed a strong correlation with the development of ORN.
In patients with head and neck carcinomas, nutritional status has been identified as a prognostic factor. Goldwaser et al. also identified it as a risk factor for ORN. In a retrospective cohort study of 82 head and neck cancer patients, they observed that the risk of ORN decreased by 50% in patients with normal BMI and by 43% in patients with a BMI between 25 and 29.9. Also, the use of steroid therapy before or after radiation treatment reduced the risk of ORN by 96%. This finding appears to support the current radiation-induced fibroatrophic process theory for ORN, as the anti-inflammatory effects of steroid therapy would limit the initial inflammatory phase of radiation therapy.
Trauma associated with dentoalveolar surgery after radiation therapy has always been considered one of the major risk factors for ORN. Despite the presence of spontaneous cases of ORN, most of ORN cases reported are preceded by tooth extractions. Utilizing pooled studies since 1986, Wahl estimated the incidence of ORN for preradiation extractions and postradiation extractions to be 3.0-3.2% and 3.1-3.5%, respectively. A 10-year single institution review of 405 patients who underwent extractions prior, during, and postradiation therapy showed an overall ORN incidence of 0.7%. Of the three ORN cases reported, two were associated with preradiation extraction and one with postradiation extractions, and no cases were identified in the treatment group where extractions were performed during therapy. Sulaiman et al. reported on 951 extractions performed on 187 patients, with 41.18% performed before radiation therapy and 57.22% after radiation therapy. They reported four cases of ORN with two patients having extractions prior to radiation therapy and two within 21 days of completion of therapy. In 2007, Chang et al. analyzed the impact of dental status and preradiation extractions on the risk of ORN in a study population of 413 patients who underwent radiation therapy for treatment of oropharyngeal carcinomas. Of those patients that developed ORN, 54% of them had preradiation extractions and 16% postradiation extractions. The conventional wisdom has been that preradiation extractions reduce the risk of ORN. These large retrospective studies certainly question the empirical practice of extraction of teeth as a preventive measure. Extraction of dentition should be based on the periodontal status of the tooth, the tumor-related prognosis, and the radiation field and dose planned. Also, the practice of indiscriminant extractions has an impact on the patient’s quality of life. Lost dentition can lead to a limited amount of functional teeth and an associated decrease in masticatory efficiency, especially in those cases where financial considerations limit restorative options.
The role of actinomyces in the pathogenesis of osteoradionecrosis has been brought into question. It was thought that the presence of actinomyces and other microorganisms constituted a surface contaminant and an opportunistic infection in a susceptible, local environment. Actinomyces are gram-positive, non–spore-forming anaerobic or microaerophilic rods and a common mucosal inhabitant in the oral cavity. Through the use of DNA-DNA hybridization techniques, scanning, and transmission electron microscopy and polymerase chain reaction (PCR) techniques, actinomyces and other anaerobic bacteria have been identified deep in ORN specimens. Actinomyces have been shown to induce bone resorption and could be a prognostic factor in ORN, as some authors have shown that their culture in bone specimens could lead to prolonged treatment and worst outcome. The role of microorganisms in the disease pathogenesis or treatment response is to be determined, but careful consideration needs to be given to instituting an effective antibiotic therapy strategy when there are clinical signs of infection in conjunction with necrosis.
Diagnostic Studies
The diagnosis of mandibular osteoradionecrosis remains primarily based on the clinical signs and symptoms present. Imaging studies allow for the confirmation of radiographic signs of necrosis and assessment of disease severity for treatment decisions and to rule out a malignant process, such as tumor recurrence or metastatic disease. The clinical changes associated with radiation therapy have a tendency to diminish the otherwise distinct radiographic features of a tumor and may be mistaken for residual or recurrent disease in some situations. A masslike effect could be present in the masseter and pterygoid muscles as manifested by an abnormal T2 signal intensity and soft tissue enhancement and thickening on magnetic resonance imaging. These soft tissue changes could also be visible on computed tomography, where swelling and enhancement could be found localized or diffusely around the areas of necrosis, making radiographic interpretation challenging .
The radiographic findings of ORN are not specific and are found in other conditions such as osteomyelitis and bisphosphonate-related osteonecrosis of the jaws. Panorex x-rays are a good screening modality but tend to underestimate the extent of the disease ( Fig. 59-1, A ). The use of computed tomography (CT) scans provides three-dimensional evaluation and better determination of the extent of necrosis to help guide management strategies. One is also able to rule out a malignant process with the use of one imaging modality. Among the osseous abnormalities found in plain films and CT, there is thinning of cortical bone, periodontal ligament widening, osteolysis, sclerosis, and sequestration ( Fig. 59-1, B and C ). Loss of bone marrow trabeculation can be seen on CT examination in addition to the predilection of the buccal cortex in manifesting radiographic changes. Air can also be present in advanced cases where an orocutaneous fistula is present.
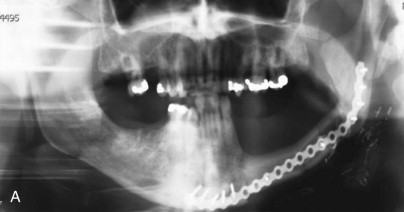
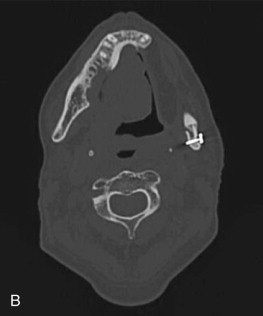
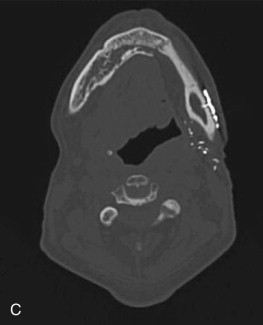
Magnetic resonance imaging (MRI) is a valuable diagnostic modality utilized in the management of ORN. It can depict early bone marrow changes prior to clinical manifestation of the disease, and the distortion resulting from dental artifacts is low. When ORN is present, bone marrow edema is depicted in MRI as reduced signal intensity in T1-weighted images and increased signal intensity in T2-weighted images when compared to normal. During treatment planning, these signal changes could be utilized for presurgical determination of the extent of necrosis, the surgical margins required, and, therefore, the reconstructive options to be considered. Functional imaging modalities also allow for means of visualizing osteoblastic and inflammatory activity in the bone. The use of classic bone scintigraphy in the diagnostic evaluation of ORN is limited by its spatial resolution and lack of specificity. In the evaluation of a patient with ORN, an increase in uptake could be representative of tumor infiltration or inflammation associated with a localized infection of dental origin. F-18 fluorodeoxyglucose (FDG) and F-18 positron emission tomography (PET) scans have been proposed as a possible tool for the assessment of disease severity in BRONJ patients because of the increase in tracer uptake in areas of inflammatory activity. Tracer activity has been correlated with prognosis in the management of head and neck carcinomas, and although no information is available on its use in ORN at this time, the use of PET/CT scans could help guide treatment decisions and predict outcomes in the future.
Disease Staging
Disease staging allows for comparison of outcome studies; it also helps guide treatment strategies and estimate prognosis. Acute and late adverse events of radiotherapy are rated, utilizing different scales. In the case of osteoradionecrosis, the lack of a uniform staging system makes comparison of the efficacy of an intervention between trials difficult. The current systems utilize a variety of criteria that could include radiographic findings, treatment response, disease progression, and the extent of necrosis. There is also a Radiation Therapy Oncology Group (RTOG) scoring criteria for bone morbidity and a comprehensive system, the Late Effects Normal Tissue Task Force subjective, objective, management, and analytic (LENT/SOMA) score, developed between European Organization for Research and Treatment of Cancer (EORTC) and the RTOG, which utilizes subjective and objective criteria in conjunction with radiographic findings and therapeutic interventions as part of the scoring system ( Tables 59-1 to 59-8 ).
| GRADE EVENT |
|---|
| 1 Bone exposure without signs of infection and persistent for at least 3 months |
| 2 Bone exposure with signs of infection or sequester and without the signs of grades 3-5 |
| 3 Bone necrosis treated with mandibular resection with a satisfactory result |
| 4 Bone necrosis with persistent problems despite mandibular resection |
| 5 Death resulting from osteoradionecrosis |
| STAGE EVENT |
|---|
| I Superficial involvement only with the necrotic bone confined to the exposed cortical bone. Soft tissue ulceration is minimal. |
| II Localized involvement with only a portion of the cortical and medullary bone being necrotic. |
III Diffuse, full thickness involvement of the mandible including the lower border.
|
| GRADE EVENT |
|---|
| I ORN confined to the alveolar bone |
| II ORN limited to the alveolar bone and or mandible above the level of mandibular alveolar canal |
| III ORN that extended to the level of the mandibular alveolar canal and ORN with a skin fistula or a pathologic fracture |
| STAGE EVENT |
|---|
| 0 Mucosal defects only |
| 1 Radiologic evidence of necrotic bone with intact mucosa |
| 2 Positive radiologic findings with denuded bone intraorally |
| 3 Clinically exposed bone, verified by imaging techniques, along with skin fistula and infection |
| STAGE EVENT |
|---|
I Resolved, healed
|
II Chronic, persistent, nonprogressive
|
III Active, progressive
|
| SCORE BONE MORBIDITY |
|---|
| 0 None |
| 1 Asymptomatic, no growth retardation, reduced bone density |
| 2 Moderate pain or tenderness, growth retardation, irregular bone sclerosis |
| 3 Severe pain or tenderness, complete arrest of bone growth, dense bone sclerosis |
| 4 Necrosis, spontaneous fracture |
| GRADE 1 | GRADE 2 | GRADE 3 | GRADE 4 | |
|---|---|---|---|---|
| Subjective | ||||
| Pain | Occasional, minimal | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
| Mastication | Difficulty with solids | Difficulty with soft foods | ||
| Denture use | Loose dentures | Inability to use dentures | ||
| Trismus | Noted but not measurable | Preventing normal eating | Difficulty eating | Inadequate oral intake |
| Objective | ||||
| Exposed bone |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


