Malignant disease can affect the oral cavity, with the most common entity being squamous cell carcinoma (SCC). Immediate intervention is necessary for an acceptable prognosis. Early detection is the most advantageous factor in decreasing disease morbidity and mortality. The combined 5-year survival rate for early-stage tumors is between 60% and 80%, but patients initially seen with late-stage disease have less than a 50% survival rate at 5 years. Innovations in imaging and chemotherapy may alter treatment planning in certain cases, but biopsy of suspicious lesions and a thorough neck examination have remained the current diagnostic modality for nearly a hundred years of attempted progressive treatment.
Etiopathogenesis/Causative Factors
Etiologic risk factors for oral squamous cell carcinoma (OSCC) are usually dependent on patients’ ethnicity and co-morbid conditions. The wide-ranging incidence of OSCC and cultural diversity throughout the world validate this fact, and the survival disparity in U.S. African Americans is exemplary. Diet, lifestyle, and available intoxicating luxuries also account for these differences.
Substance Abuse
Long-term abuse of tobacco is the most common etiologic factor for OSCC. Alcohol, oral hygiene (specifically, polymicrobial supragingival plaque ), and nutritional deficits are statistical complementary factors along with tobacco. The molecular mechanism for the initiation of cancer is believed to be a DNA insult from nitrosamine compounds and inhibition of repair from alcohol use. These factors account for nearly 75% of cases of OSCC worldwide. Individuals with greater than a 30 pack per year history of tobacco abuse have a four-fold greater propensity for the development of OSCC. Only smokers who have quit for more than 16 years have the same risk as non-smokers. Smokers who continue to smoke have a 40% chance for the development of a second primary. De novo detection of cancer in these patients could optimize survival and is being investigated with molecular markers. Presently, 23% of teens smoke in the United States.
Many southern Asian populations chew betel quid ( Fig. 53-1 ). The stimulant, socially acceptable as coffee, is associated with oral cancer with a predilection for the buccal mucosa in this subcontinent, thus hinting at a genetic predisposition. Recent studies suggest that targeting epidermal growth factor receptor (EGFR) may benefit patients with this habit because of overexpression of EGFR. Many of these patients also have submucous fibrosis and, when combined with betel quid, yields an incidence of cancer from malignant transformation surpassing 7%.

Co-Morbid Conditions
Oral lichen planus, a leukoplakia and autoimmune inflammatory disease, does have the propensity to progress to malignancy. Other disease, such as Fanconi anemia, a genetic disorder with an increased incidence in patients with solid tumors, graft-versus-host disease in immunosuppressed patients, and syphilis are also prone to malignant conversion. All predispositions are a result of chronic inflammation and subsequent high cell turnover by immune-mediated cellular proliferation and attempted apoptosis—both known initiators of precancerous lesions.
Environment
Widespread knowledge of smoking’s ill effects led to U.S. environmental protection laws forbidding smoking in many public spaces. Secondhand smoke was and, in some places, still is a ubiquitous insult. However, the likelihood of such governmental policy causing a decline in the statistical incidence of carcinoma cannot be known because of the latent onset of oral cancer. Other potential air pollutants, such as organic and inorganic particulates (e.g., nitrosamines, polycyclic aromatic hydrocarbons, and viruses), are associated with oral cancer in workplaces. Nonetheless, few regulations are instituted except for mandatory donning of safety masks to possibly decrease the transmission of airborne noxious particles.
Unknown
A subset of cancer patients have no risk factors (i.e., never smoked and never drank). This group is more often young women who are likely to be serologically positive for human papillomavirus type 16 and to have early-stage carcinoma of the lateral border of the tongue diagnosed. This population represented 18% of oral cancer patients in the United States, an increase from 4% in 1971. Short-term abuse—or the “binge” abuse of tobacco and alcohol that commonly exists in younger subcultures—may be accountable but has yet to be proved detrimental.
Pathologic Anatomy
Spread of OSCC among anatomic planes can be cryptic, especially in recurrent cases. The oral cavity and thin, non-irradiated necks can easily be investigated for local occurrence and regional metastasis, respectively. However, some clinical disease manifestations do need extra attention because of either anatomic uniqueness or surgical access. This review of pathologic anatomy is for the oral oncologic surgeon and is by no means a comprehensive discussion. Furthermore, although the most common sites for oral cancer are the lateral aspect of the tongue and the floor of the mouth, the subunits in the following sections have unique surgical implications that require special attention.
Coffin Corner
The alveololingual sulcus, a “coffin corner” not unlike the blind niche found in the corner landing of a Victorian house stairwell, is a poorly accessed mucosal surface. Without the use of anterior retraction and digital palpation of the tongue, the region may be neglected ( Fig. 53-2 ). This area should be thoroughly evaluated in patients with floor of the mouth or lateral tongue lesions. If cancer abuts the ramus, the surgeon should not hesitate to perform periosteal stripping by elevating the periosteum and then visually inspecting for macroinvasion.
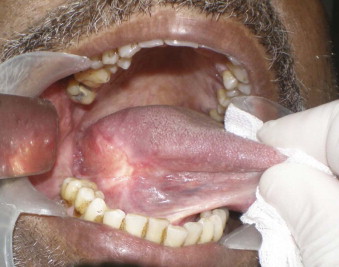
Bone Invasion
Lesions extending onto the maxillary and mandibular alveoli are highly susceptible to osseous invasion. Pathologists recognize two distinct patterns: (1) an invasive histology dotted with islands of bone and finger-like projections of abnormal epithelium that advance independently of the cancellous spaces and (2) an erosive type characterized by a broad advancing front projecting into the cancellous spaces with the aid of osteoclasts (e.g., verrucous carcinoma).
Invasion of OSCC into bone may occur from several routes: oral cavity occlusal route, mental or inferior alveolar foramen (neurotrophic), secondary tumors in the neck through the lower border, cortical bone defects in an edentulous ridge, periodontal membrane in the dentate arch, and the attached gingiva. This incorrect paradigm led to more radical mandibular “rim” resection that included the inferior alveolar nerve for non-bulky tumors.
The greatest predilection is direct invasion, and remotely, neurotrophic-, marrow space–, or periodontal ligament–associated invasion is possible. Computed tomography (CT) statistically misses 27% of these cases and is thus not considered a definitive evaluation. Therefore, periosteal stripping lends credence to the non-partisan and insidious spread of cancer to bone. Edentulous arches are not more susceptible than dentate ones but may lend themselves to more complicated reconstruction if a defect in continuity exists after resection.
Pterygopalatine Space
Poor access and numerous vital structures may hinder isolation and prevent adequate resection of this retromaxillary region. Worthington previously documented the following passages as testaments to the area’s obscure surgical difficulty :
“An area seen with difficulty, round dark corners …” and “… the posterior section is carried out blind and by blunt leverage.” *
“… critical margin in a cavity filled with blood within several millimetres of the internal carotid artery.” †
* Crocket JD: Surgical approach to the back of the maxilla, Br J Surg 50:819-821, 1963.
† Dingman L, Conley J: Lateral approach to the pterygomaxillary region, Ann Otol Rhinol Laryngol 79:967, 1970.
The complexity of nerves, skull base, and multiple conduits necessitates accurate preoperative planning, which is best accomplished with the aid of magnetic resonance imaging (MRI) and positron emission tomography (PET). Diagrammatic representation of the fossa may assist in performing en bloc resection ( Fig. 53-3 ). The space’s pathology or resected margins often originate from the antrum or buccal space. The buccal space and its extension (e.g., masseteric space, infratemporal fossa, middle cranial fossa) are extremely vulnerable in late-stage buccal OSCC, and preoperative imaging of radiographic changes in the infratemporal fossa should differentiate vascular insufficiency from extension of OSCC.
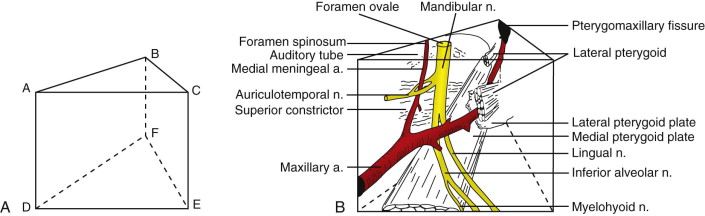
Attempting an “extended” maxillectomy by perioral resection (i.e., transpalatal or transantral approach) for OSCC extending into the pterygopalatine fossa is not possible because wide access must be gained to achieve negative margins and vascular hemostasis. Extirpation may be initiated from several other vantage points: transzygomatic, transcranial, transmandibular, facial translocation, or a combination of these techniques. The anterior transmandibular approach using the Dingman-Conley split lip, with or without marginal or segmental mandibulotomy for mandibular disease, provides proper exposure, whereas a Weber-Fergusson access, with subciliary or medial canthal extension if needed, is best suited for antral disease; a Barbosa design may be necessary for extensive antral disease. The surgeon may modify skin incisions by using chevrons to take advantage of the relaxed skin tension lines of the face to achieve optimal esthetic outcomes.
Submandibular Region
The submandibular gland (SMG) and its vessels and adjacent anatomy are responsible for the complexity of level IB. The submandibular duct and hypoglossal and lingual nerves can easily be accessed with gentle downward traction of the SMG and ligation of the periglandular vessels. Recognition and isolation of these numerous vessels are important for prevention of postoperative oozing and selection of recipient vessels for microsurgical anastomosis. The SMG’s primary arterial cascade is the facial and submental arteries, but there are variations from the external carotid, lingual, and deep lingual branches. Its main venous supply consists of the anterior facial, venae comitantes of the facial arteries, and gland hilum veins, but the external jugular veins, anterior jugular veins, and mental veins may be contributory.
Fascia
Knowledge of the head and neck fascia and its spaces is essential, and it is a voluminous subject. The four layers of fascia consist of the superficial fascia and the superficial, middle, and deep layers of the deep cervical fascia. Its primary importance pertains to preservation of the marginal mandibular nerve and perifacial node dissection near the mandibular line (discussed in the following sections).
Marginal Mandibular Nerve
This motor branch of the facial nerve to the lip depressor ( depressor anguli oris ) is a critical structure and can be injured during cervical access, perifacial node dissection, and elevation of lower cheek flaps. The nerve lies within or deep to the superficial layer of the deep cervical fascia. Almost 1 in 10 surgical patients will have at least one branch of the marginal mandibular nerve located within 1 cm of the inferior border of the mandible lateral to the facial artery; medial to the facial artery, all rami are found above the inferior border of the mandible; and nearly all (95%) marginal mandibular nerves run superficial to the anterior facial and retromandibular veins. To protect the nerve, all cervical incisions are made at least 2 cm caudal to the inferior border of the mandible, and all dissections are performed below the veins (Hayes-Martin maneuver).
Cervical Lymphatics
Henri Rouvière schematically described the lymphatic drainage of the head and neck as two concentric narrowing funnels draining caudally to the thoracic duct (left) and lymphatic duct (right). This paradigm is oversimplistic but is still taught in schools today. Regional metastases from the oral cavity often drain to Robbins levels I to III, hence the rationale for supraomohyoid neck dissection. Skip metastases are possible, and the tongue and soft palate are often discovered to have bilateral regional metastases on final pathologic evaluation. Table 53-1 illustrates the anatomic levels of the neck and their importance.
| NECK REGION | NODAL LEVEL | IMPORTANCE |
|---|---|---|
| Submental triangle | Level Ia | Submental arteries as salvage for island flap |
| Submandibular triangle | Level Ib | Sentinel lymphatic basin for OSCC, node of Stahr |
| Para–internal jugular vein (infra–skull base to suprahyoid) | Level IIa | Sentinel lymphatic basin for most OSCC, jugulodigastric node (Küttner’s node) |
| Posterior to the spinal accessory nerve | Level IIb | Dissect for N+ only (comprehensive ND only) |
| Para–internal jugular vein (infrahyoid to supracricoid) | Level III | Inferior extent of supraomohyoid ND, jugulo-omohyoid node (Poirier’s node) |
| Para–internal jugular vein (infracricoid to supraclavicular) | Level IV | Possibly occult in lateral tongue cancer (extended ND only), Virchow’s node * |
| Posterior triangle | Level V | Dissect for N+ neck (comprehensive ND only) |
| Perifacial node | Above superficial layer of deep cervical fascia | Risk for marginal mandibular nerve injury |
Predictable drainage of the oral cavity to the first echelon of the lymphatic basin does exist. However, as a result of data from large clinical outcome studies and the ability of lymphoscintigraphy to map sentinel nodes, surgeons now recognize that drainage can be on an individual basis. Skip metastases to level IV in lateral tongue SCC and retropharyngeal drainage of the soft palate follow this paradigm. Another caveat lector is that previously operated necks may have hitherto undiagnosed, nascent, or recurrent metastases. Therefore, lymphatic drainage can be unpredictable after surgery, and clinically positive (cN+) or negative (cN0) nodes with micrometastases may go undetected.
Radiologists combine nodal critical size and morphology to determine “suggestive” cervical adenopathy. Suspicion for regional metastases is high in the setting of OSCC if nodes display a central hypointensity consistent with central necrosis; if they are round and not kidney bean shaped, which represents expansion; if the surrounding fascial plane is obliterated, which signifies tissue necrosis or fixation; if their dimensions are greater than 15 mm at level II and greater than 10 mm elsewhere; or if a spiculated periphery indicative of extracapsular spread is present. This last characteristic is a significant poor prognostic indicator for OSCC.
Perifacial Node
The perifacial, perivascular, or supramandibular facial node lies just superior to the inferior mandibular bone margin near the antegonial notch where the facial artery and vein pass cephalad. Adjacent regional metastasis to the perifacial node is relatively highly likely if level Ib is positive—nearly 35% and 27% of node-positive and node-negative necks, respectively. To gain access to the node, the superficial layer of the deep cervical fascia must be violated and dissection must proceed in a more superficial direction. Taking care to not damage the marginal mandibular nerve with this maneuver is imperative, and a risk/benefit decision must be made before dissection of occult disease. Node-positive patients who require comprehensive neck dissection and have aggressive buccal and mandibular mucosal lesions should be candidates for perifacial node removal.
Pathologic Anatomy
Spread of OSCC among anatomic planes can be cryptic, especially in recurrent cases. The oral cavity and thin, non-irradiated necks can easily be investigated for local occurrence and regional metastasis, respectively. However, some clinical disease manifestations do need extra attention because of either anatomic uniqueness or surgical access. This review of pathologic anatomy is for the oral oncologic surgeon and is by no means a comprehensive discussion. Furthermore, although the most common sites for oral cancer are the lateral aspect of the tongue and the floor of the mouth, the subunits in the following sections have unique surgical implications that require special attention.
Coffin Corner
The alveololingual sulcus, a “coffin corner” not unlike the blind niche found in the corner landing of a Victorian house stairwell, is a poorly accessed mucosal surface. Without the use of anterior retraction and digital palpation of the tongue, the region may be neglected ( Fig. 53-2 ). This area should be thoroughly evaluated in patients with floor of the mouth or lateral tongue lesions. If cancer abuts the ramus, the surgeon should not hesitate to perform periosteal stripping by elevating the periosteum and then visually inspecting for macroinvasion.
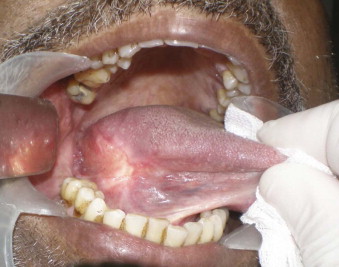
Bone Invasion
Lesions extending onto the maxillary and mandibular alveoli are highly susceptible to osseous invasion. Pathologists recognize two distinct patterns: (1) an invasive histology dotted with islands of bone and finger-like projections of abnormal epithelium that advance independently of the cancellous spaces and (2) an erosive type characterized by a broad advancing front projecting into the cancellous spaces with the aid of osteoclasts (e.g., verrucous carcinoma).
Invasion of OSCC into bone may occur from several routes: oral cavity occlusal route, mental or inferior alveolar foramen (neurotrophic), secondary tumors in the neck through the lower border, cortical bone defects in an edentulous ridge, periodontal membrane in the dentate arch, and the attached gingiva. This incorrect paradigm led to more radical mandibular “rim” resection that included the inferior alveolar nerve for non-bulky tumors.
The greatest predilection is direct invasion, and remotely, neurotrophic-, marrow space–, or periodontal ligament–associated invasion is possible. Computed tomography (CT) statistically misses 27% of these cases and is thus not considered a definitive evaluation. Therefore, periosteal stripping lends credence to the non-partisan and insidious spread of cancer to bone. Edentulous arches are not more susceptible than dentate ones but may lend themselves to more complicated reconstruction if a defect in continuity exists after resection.
Pterygopalatine Space
Poor access and numerous vital structures may hinder isolation and prevent adequate resection of this retromaxillary region. Worthington previously documented the following passages as testaments to the area’s obscure surgical difficulty :
“An area seen with difficulty, round dark corners …” and “… the posterior section is carried out blind and by blunt leverage.” *
“… critical margin in a cavity filled with blood within several millimetres of the internal carotid artery.” †
* Crocket JD: Surgical approach to the back of the maxilla, Br J Surg 50:819-821, 1963.
† Dingman L, Conley J: Lateral approach to the pterygomaxillary region, Ann Otol Rhinol Laryngol 79:967, 1970.
The complexity of nerves, skull base, and multiple conduits necessitates accurate preoperative planning, which is best accomplished with the aid of magnetic resonance imaging (MRI) and positron emission tomography (PET). Diagrammatic representation of the fossa may assist in performing en bloc resection ( Fig. 53-3 ). The space’s pathology or resected margins often originate from the antrum or buccal space. The buccal space and its extension (e.g., masseteric space, infratemporal fossa, middle cranial fossa) are extremely vulnerable in late-stage buccal OSCC, and preoperative imaging of radiographic changes in the infratemporal fossa should differentiate vascular insufficiency from extension of OSCC.
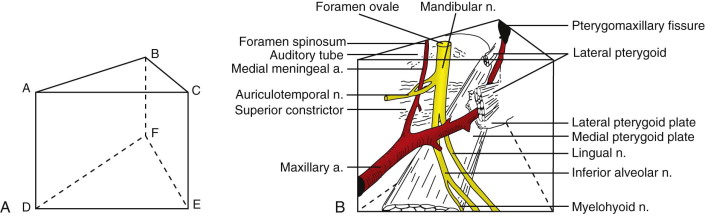
Attempting an “extended” maxillectomy by perioral resection (i.e., transpalatal or transantral approach) for OSCC extending into the pterygopalatine fossa is not possible because wide access must be gained to achieve negative margins and vascular hemostasis. Extirpation may be initiated from several other vantage points: transzygomatic, transcranial, transmandibular, facial translocation, or a combination of these techniques. The anterior transmandibular approach using the Dingman-Conley split lip, with or without marginal or segmental mandibulotomy for mandibular disease, provides proper exposure, whereas a Weber-Fergusson access, with subciliary or medial canthal extension if needed, is best suited for antral disease; a Barbosa design may be necessary for extensive antral disease. The surgeon may modify skin incisions by using chevrons to take advantage of the relaxed skin tension lines of the face to achieve optimal esthetic outcomes.
Submandibular Region
The submandibular gland (SMG) and its vessels and adjacent anatomy are responsible for the complexity of level IB. The submandibular duct and hypoglossal and lingual nerves can easily be accessed with gentle downward traction of the SMG and ligation of the periglandular vessels. Recognition and isolation of these numerous vessels are important for prevention of postoperative oozing and selection of recipient vessels for microsurgical anastomosis. The SMG’s primary arterial cascade is the facial and submental arteries, but there are variations from the external carotid, lingual, and deep lingual branches. Its main venous supply consists of the anterior facial, venae comitantes of the facial arteries, and gland hilum veins, but the external jugular veins, anterior jugular veins, and mental veins may be contributory.
Fascia
Knowledge of the head and neck fascia and its spaces is essential, and it is a voluminous subject. The four layers of fascia consist of the superficial fascia and the superficial, middle, and deep layers of the deep cervical fascia. Its primary importance pertains to preservation of the marginal mandibular nerve and perifacial node dissection near the mandibular line (discussed in the following sections).
Marginal Mandibular Nerve
This motor branch of the facial nerve to the lip depressor ( depressor anguli oris ) is a critical structure and can be injured during cervical access, perifacial node dissection, and elevation of lower cheek flaps. The nerve lies within or deep to the superficial layer of the deep cervical fascia. Almost 1 in 10 surgical patients will have at least one branch of the marginal mandibular nerve located within 1 cm of the inferior border of the mandible lateral to the facial artery; medial to the facial artery, all rami are found above the inferior border of the mandible; and nearly all (95%) marginal mandibular nerves run superficial to the anterior facial and retromandibular veins. To protect the nerve, all cervical incisions are made at least 2 cm caudal to the inferior border of the mandible, and all dissections are performed below the veins (Hayes-Martin maneuver).
Cervical Lymphatics
Henri Rouvière schematically described the lymphatic drainage of the head and neck as two concentric narrowing funnels draining caudally to the thoracic duct (left) and lymphatic duct (right). This paradigm is oversimplistic but is still taught in schools today. Regional metastases from the oral cavity often drain to Robbins levels I to III, hence the rationale for supraomohyoid neck dissection. Skip metastases are possible, and the tongue and soft palate are often discovered to have bilateral regional metastases on final pathologic evaluation. Table 53-1 illustrates the anatomic levels of the neck and their importance.
| NECK REGION | NODAL LEVEL | IMPORTANCE |
|---|---|---|
| Submental triangle | Level Ia | Submental arteries as salvage for island flap |
| Submandibular triangle | Level Ib | Sentinel lymphatic basin for OSCC, node of Stahr |
| Para–internal jugular vein (infra–skull base to suprahyoid) | Level IIa | Sentinel lymphatic basin for most OSCC, jugulodigastric node (Küttner’s node) |
| Posterior to the spinal accessory nerve | Level IIb | Dissect for N+ only (comprehensive ND only) |
| Para–internal jugular vein (infrahyoid to supracricoid) | Level III | Inferior extent of supraomohyoid ND, jugulo-omohyoid node (Poirier’s node) |
| Para–internal jugular vein (infracricoid to supraclavicular) | Level IV | Possibly occult in lateral tongue cancer (extended ND only), Virchow’s node * |
| Posterior triangle | Level V | Dissect for N+ neck (comprehensive ND only) |
| Perifacial node | Above superficial layer of deep cervical fascia | Risk for marginal mandibular nerve injury |
Predictable drainage of the oral cavity to the first echelon of the lymphatic basin does exist. However, as a result of data from large clinical outcome studies and the ability of lymphoscintigraphy to map sentinel nodes, surgeons now recognize that drainage can be on an individual basis. Skip metastases to level IV in lateral tongue SCC and retropharyngeal drainage of the soft palate follow this paradigm. Another caveat lector is that previously operated necks may have hitherto undiagnosed, nascent, or recurrent metastases. Therefore, lymphatic drainage can be unpredictable after surgery, and clinically positive (cN+) or negative (cN0) nodes with micrometastases may go undetected.
Radiologists combine nodal critical size and morphology to determine “suggestive” cervical adenopathy. Suspicion for regional metastases is high in the setting of OSCC if nodes display a central hypointensity consistent with central necrosis; if they are round and not kidney bean shaped, which represents expansion; if the surrounding fascial plane is obliterated, which signifies tissue necrosis or fixation; if their dimensions are greater than 15 mm at level II and greater than 10 mm elsewhere; or if a spiculated periphery indicative of extracapsular spread is present. This last characteristic is a significant poor prognostic indicator for OSCC.
Perifacial Node
The perifacial, perivascular, or supramandibular facial node lies just superior to the inferior mandibular bone margin near the antegonial notch where the facial artery and vein pass cephalad. Adjacent regional metastasis to the perifacial node is relatively highly likely if level Ib is positive—nearly 35% and 27% of node-positive and node-negative necks, respectively. To gain access to the node, the superficial layer of the deep cervical fascia must be violated and dissection must proceed in a more superficial direction. Taking care to not damage the marginal mandibular nerve with this maneuver is imperative, and a risk/benefit decision must be made before dissection of occult disease. Node-positive patients who require comprehensive neck dissection and have aggressive buccal and mandibular mucosal lesions should be candidates for perifacial node removal.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


