The status of the cervical lymph nodes is the most important prognostic factor in squamous cell carcinoma (SCC) of the head and neck, and the clinical significance of regional nodal metastases has long been recognized. The overall survival rate decreases by approximately 50% in patients with metastases to the cervical lymph nodes. Despite the progress made in patient education and early detection of SCC of the oral cavity, approximately 40% of patients will initially be found to have evidence of regional nodal metastasis. Therefore, management of the cervical lymphatics is an important component of the overall treatment of patients with head and neck cancer.
Anatomy
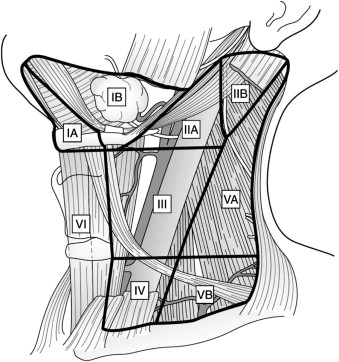
The head and neck drain into an extensive network of cervical lymphatics, and earlier studies demonstrated that this drainage pattern usually occurs in a predictable manner for each site. Knowledge of the anatomy of the regional lymphatic system is therefore important for clinicians treating patients with head and neck cancer. To facilitate communication among the various disciplines involved, the Head and Neck Service at the Memorial-Sloan Kettering Cancer Center proposed a system in which the cervical lymphatic system is divided into levels corresponding to certain clinical and radiographic landmarks. Further modifications of this system resulted in a widely accepted method of defining the levels of the cervical lymphatic network that is endorsed by the American Head and Neck Society and the American Academy of Otolaryngology ( Fig. 54-1 , Box 54-1 ).
- •
Level I: Extends from the hyoid bone inferiorly to the inferior border of the mandible superiorly and bounded by the digastric muscle. The submental and submandibular nodes lie in this level. It is further subdivided into
- •
Level Ia (submental group): Bounded by the anterior bellies of the digastric muscle and extends from the hyoid bone to the symphysis of the mandible.
- •
Level Ib (submandibular group): Triangular area bounded by the posterior and anterior bellies of the digastric muscle and the body of the mandible. Includes the lymph nodes along the facial artery adjacent to the submandibular gland.
- •
- •
Level II (upper jugular group): Contains nodes that surround the upper third of the internal jugular vein and the spinal accessory nerve. The jugulodigastric node lies here, which is the most common node for metastasis of squamous cell carcinoma of the oral cavity. This level can be subdivided based on the spinal accessory nerve:
- •
Level IIa: Extends from the base of the skull superiorly to the hyoid bone (radiographic) or carotid bifurcation (clinical) inferiorly. It is bounded anteriorly by the lateral aspect of the sternohyoid muscle and posteriorly by a vertical plane defined by the course of the spinal accessory nerve.
- •
Level IIb: Extends from the base of the skull superiorly to the hyoid bone (radiographic) or carotid bifurcation (clinical) inferiorly. It is bounded anteriorly by a vertical plane defined by the course of the spinal accessory nerve and posteriorly by the lateral edge of the sternocleidomastoid muscle.
- •
- •
Level III (mid-jugular group): Contains nodes that encompass the middle third of the internal jugular vein. It is bounded superiorly by the hyoid bone (radiographic) or the carotid bifurcation (clinical) and inferiorly by the cricoid cartilage (radiographic) or the omohyoid muscle (clinical). Anteriorly, it extends to the lateral aspect of the sternohyoid muscle, and its posterior limit is the lateral edge of the sternocleidomastoid muscle. The jugulo-omohyoid node lies at this level.
- •
Level IV (lower jugular group): Contains nodes surrounding the inferior third of the internal jugular vein. It extends from the inferior border of level III to the clavicle. Anteriorly it is bounded by the lateral aspect of the sternohyoid muscle, and posteriorly it is bounded by the lateral edge of the sternocleidomastoid muscle. Of note, this level is rarely involved in oral cavity squamous cell carcinoma unless one of the higher-echelon nodes is involved ( 1 ).
- •
Level V (posterior triangle group): Contains nodes around the inferior portion of the spinal accessory nerve and transverse cervical vessels. Similar to level IV nodes, these nodes are not usually involved in oral cavity squamous cell carcinoma unless there is metastasis to the upper-echelon nodes ( 1 ). It can be subdivided into
- •
Level Va: The superior border is the junction of the sternocleidomastoid and trapezius muscles. The inferior border is determined by a horizontal line drawn at the level of the cricoid cartilage. The anterior border is the lateral edge of the sternocleidomastoid muscle. Posteriorly, it extends to the medial aspect of the trapezius muscle.
- •
Level Vb: The superior border is determined by a horizontal line drawn at the level of the cricoid cartilage. The clavicle represents the inferior border. The anterior border is the lateral edge of the sternocleidomastoid muscle. Posteriorly, it extends to the medial aspect of the trapezius muscle.
- •
- •
Level VI (central compartment group): Extends from the hyoid bone superiorly to the suprasternal notch inferiorly. It lies between the medial borders of the carotid sheaths bilaterally. These nodes are rarely involved in oral cavity squamous cell carcinoma.
- •
Level VII (superior mediastinal group): Extends from the suprasternal notch to the innominate artery and includes the lymph nodes in the anterior superior mediastinum. Rarely involved in oral cavity squamous cell carcinoma.
Classification of Neck Dissections
Robbins and co-workers defined a classification system outlining the various types of neck dissection.
Radical Neck Dissection
Radical neck dissection (RND) involves the en bloc removal of all ipsilateral lymph nodes from levels I through V, along with the ipsilateral spinal accessory nerve (SAN), internal jugular vein (IJV), and sternocleidomastoid muscle (SCM).
Modified Radical Neck Dissection
When one or more non-lymphatic structures are preserved during the dissection, the procedure is termed a modified radical neck dissection (MRND). The basis for this modification is that the lymph node–containing tissues lie within the cervical fascial planes surrounding the SCM, IJV, and SAN and that these structures can be preserved if they are not involved with tumor by skeletonizing them during the dissection. MRND can be subclassified as follows: type I MRND preserves the SAN; type II MRND preserves the SAN and IJV; and type III MRND preserves the SAN, IJV, and SCM.
Selective Neck Dissection
In selective neck dissection (SND), one or more lymph node groups are preserved during cervical lymphadenectomy that are routinely removed with RND. The lymph node groups that are removed are dependent on the predictable patterns of metastases from the primary site. The levels of lymph nodes removed are identified (e.g., SND levels I to III).
Extended Neck Dissection
With the removal of one or more lymph node groups or non-lymphatic structures, or both, that are not usually involved in RND, the procedure is termed extended neck dissection. Examples of lymph node groups include the parapharyngeal, paratracheal, and superior mediastinal nodes. Examples of non-lymphatic structures include the carotid artery, hypoglossal nerve, and paraspinal muscles.
Classification of Neck Dissections
Robbins and co-workers defined a classification system outlining the various types of neck dissection.
Radical Neck Dissection
Radical neck dissection (RND) involves the en bloc removal of all ipsilateral lymph nodes from levels I through V, along with the ipsilateral spinal accessory nerve (SAN), internal jugular vein (IJV), and sternocleidomastoid muscle (SCM).
Modified Radical Neck Dissection
When one or more non-lymphatic structures are preserved during the dissection, the procedure is termed a modified radical neck dissection (MRND). The basis for this modification is that the lymph node–containing tissues lie within the cervical fascial planes surrounding the SCM, IJV, and SAN and that these structures can be preserved if they are not involved with tumor by skeletonizing them during the dissection. MRND can be subclassified as follows: type I MRND preserves the SAN; type II MRND preserves the SAN and IJV; and type III MRND preserves the SAN, IJV, and SCM.
Selective Neck Dissection
In selective neck dissection (SND), one or more lymph node groups are preserved during cervical lymphadenectomy that are routinely removed with RND. The lymph node groups that are removed are dependent on the predictable patterns of metastases from the primary site. The levels of lymph nodes removed are identified (e.g., SND levels I to III).
Extended Neck Dissection
With the removal of one or more lymph node groups or non-lymphatic structures, or both, that are not usually involved in RND, the procedure is termed extended neck dissection. Examples of lymph node groups include the parapharyngeal, paratracheal, and superior mediastinal nodes. Examples of non-lymphatic structures include the carotid artery, hypoglossal nerve, and paraspinal muscles.
Management of Node-Positive Necks
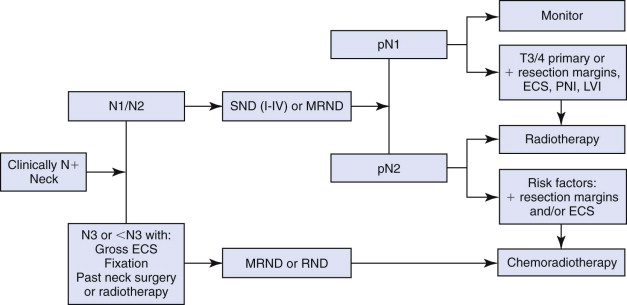
Oncologic principles dictate that the management of regional cervical nodal metastasis from head and neck cancer should involve removal of the diseased nodal groups and comprehensive clearance of all the remaining ipsilateral lymphatic groups.
RND was the initial form of comprehensive cervical lymphadenectomy and was popularized by Crile in 1906. Further contributions by Martin led to acceptance of RND as the standard operation for cervical lymphadenectomy in head and neck cancer, and it was the most common type of neck dissection performed until the 1960s. It has been proved to improve survival and is still considered the gold standard for management of metastasis to the cervical lymphatics.
However, because significant functional and cosmetic morbidity can be associated with removal of the SCM, IJV, and SAN, modifications in RND were performed in the form of the MRND, as pioneered by Suarez, Bocca, Byers, and their colleagues. Subsequent studies have demonstrated that MRND is as effective for regional control as RND in treating metastases to the cervical lymphatics in head and neck cancer. MRND became the accepted standard for surgical management of cervical disease, although RND still has a role in cases in which the SAN, IJV, or SCM is involved.
Some surgeons advocate the use of SND even in the presence of nodal disease, although this contradicts the oncologic principles of en bloc excision of all ipsilateral cervical lymphatics. This remains a controversial topic. The reasoning for the use of SND in node-positive necks is based on studies demonstrating the patterns of metastasis to the cervical lymphatics from various primary sites. For instance, cancers originating from the oropharynx, hypopharynx, and larynx have a predilection to metastasize to levels II to IV. Oral cavity cancers have a tendency to metastasize to levels I to III. Furthermore, for oral cavity cancers that have spread to level I, II, or III, the risk for involvement of level IV increases from 3% to 17%; however, the risk for level V involvement increases from only 1% to 6%. Therefore, for oral cavity cancers that have spread to the neck, surgical excision of levels I to IV will remove the majority of nodal metastases, and multiple studies have demonstrated good locoregional control rates with SND for node-positive necks. Typically, SND of levels I to III is performed for oral cavity cancer and SND of levels II to IV for oropharyngeal, hypopharyngeal, and laryngeal cancer. If there is a suspicious node involved at the lowest level, it is recommended that the dissection be extended to include the next level. However, this may not apply to nodes involved in level IV because there is evidence that lymphatic flow does not occur from the jugular chain to the posterior triangle. It is important to note that SND is not indicated in certain patients, specifically, those with massive adenopathy, clinical evidence of gross extracapsular spread, nodal fixation, previous neck surgery, or previous neck irradiation.
Currently, MRND is the mainstay of surgical treatment of node-positive necks. However, there is evidence that the use of SND may be appropriate in select cases. A comprehensive review by Nikolarakos and co-authors discusses the management of node-positive necks in patients with oral SCC ( Fig. 54-2 ).
Management of Node-Negative Necks
Treatment of a clinically node-negative neck (cN0) in a patient with oral SCC presents a significant dilemma to the head and neck oncologic surgeon. As mentioned previously, the presence of neck metastases reduces the 5-year survival rate by almost 50%. Studies have demonstrated that occult metastasis occurs in approximately 20% to 45% of patients who were clinically staged as N0. This has led to the option of performing elective neck dissection (END) in patients with no clinical evidence of nodal metastases. However, this may lead to 55% to 80% of patients undergoing unnecessary neck dissections, along with the associated morbidity, particularly postoperative shoulder dysfunction. Therefore, accurate staging of the regional lymph nodes is important for prognostic and diagnostic purposes. Currently, the debate centers around determining which patients will benefit from elective treatment of a cN0 neck and which patients can be managed with a close observation, “wait-and-see” approach. This section discusses the various management issues and options currently available.
Diagnostic Modalities
The decision of when to electively treat a cN0 neck might be facilitated if there were an accurate, non-invasive method of determining the presence of occult nodal metastases.
The use of clinical examination alone to detect occult nodal metastasis has been proved to be inadequate, even by experienced clinicians. Studies evaluating the effectiveness of clinical palpation of the neck for nodal metastases have reported specificities and sensitivities ranging from 60% to 80%. One study demonstrated the negative predictive value (NPV) of clinical examination to be only 55% in assessing for nodal metastasis from oral tongue cancer.
Various anatomic imaging techniques have been used in an attempt to detect the 20% to 45% of cN0 necks that may harbor occult nodal metastases. The common techniques used are computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound. However, the radiologic changes associated with nodal metastasis (e.g., central necrosis, cystic changes) are rarely seen in nodes with microscopic, occult disease. Furthermore, the orientation of the long axis of individual nodes is variable in different regions of the neck, thereby making it difficult to accurately identify their dimensions radiologically. Therefore, distinguishing between a reactive lymph node and one that is infiltrated with tumor can be difficult.
Contrast-enhanced CT and MRI have been shown to produce similar results in assessing for occult nodal metastases, with sensitivities ranging from 56% to 85% and specificities from 47% to 95%.
Ultrasound can be advantageous because it can readily be used to guide fine-needle aspiration biopsy (UGFNAB). However, for an N0 neck, studies have shown that the sensitivity of UGFNAB ranges from just 48% to 76%. One study from a group advocating the use of UGFNAB along with the “wait-and-see” approach for the management of N0 necks found that lymph node metastasis developed in 21% of the patients during follow-up, with a salvage rate of 79% after therapeutic neck dissection and postoperative radiation therapy, for an overall regional control rate of 88%. The disparity between the studies may be due to operator experience, with UGFNAB possibly requiring a high level of technical skill.
Positron emission tomography (PET) in combination with CT is a relatively new technology that is being used to evaluate patients with head and neck SCC. PET scans by themselves have been shown to be more sensitive than CT or MRI in identifying primary tumors and metastatic neck disease. By combining PET with CT, the hope is to obtain better anatomic localization of the lesions and thus improve the sensitivity of the study. However, a prospective study evaluating the use of PET/CT for detecting regional nodal metastasis in cN0 necks demonstrated a sensitivity for detection of disease of 67% and a specificity of 95%. Based on the findings, the authors concluded that they do not recommend the use of PET/CT alone in the management of cN0 necks. Furthermore, the radiation exposure from PET/CT scans is associated with a small, but real risk for the development of a second cancer.
Thus, although these modalities may be useful adjuncts in the diagnostic management of cN0 necks in patients with head and neck SCC, they are not sensitive enough to replace END for histopathologic staging as the gold standard for staging of head and neck SCC.
Sentinel Lymph Node Biopsy
Sentinel lymph node biopsy (SLNB) is described as an intermediate option for the management of cN0 necks, one that is between the close observation (“wait and see”) and elective treatment groups. It is potentially less morbid than neck dissection and can effectively stage the neck. SLNB is a concept based on the principle that cancers spread to regional lymph nodes in a predictable manner and that if the upper-echelon nodes can be identified and analyzed accurately, this will give valuable information regarding the status of the rest of the neck. Radioactive tracers or blue dye (or both) is injected around the periphery of the primary tumor site, and with the use of lymphoscintigraphy and intraoperative gamma probes, the sentinel nodes are identified and harvested. These nodes then undergo comprehensive histopathologic analysis involving routine hematoxylin-eosin staining and immunohistochemistry, and the assumption is that if these nodes are negative for any tumor deposits, the rest of the neck is less likely to harbor any further metastases.
The concept of SLNB was described in the 1960s, and its efficacy in the treatment of malignant melanoma and breast cancer has been well documented. This tool has recently been applied to the management of cN0 necks in patients with head and neck SCC, and multiple pilot studies have demonstrated promising results.
A European prospective, multicenter trial investigated 227 patients who underwent SLNB alone or in conjunction with END. These patients had T1/T2 SCC of the oral cavity or oropharynx and were clinically staged as N0. SLNB was successful in identifying a sentinel node in 93% of cases, with a rate of 83% for tumors of the floor of the mouth and 96% for all other sites. The difference may be due to the close proximity of tumors in the floor of the mouth to the draining nodes. An average of 2.8 nodes were identified per patient. Thirty-four percent of the cases were upstaged, and the sensitivity for the SLNB-alone group was 91%. They concluded, based on these preliminary results, that SLNB can be used alone to clinically stage N0 necks in most patients with T1/T2 SCC of the oral cavity and oropharynx, with those of the floor of the mouth being more difficult.
A subsequent North American prospective, multicenter trial investigated 140 patients with T1/T2 oral SCC and cN0 necks. These patients underwent SLNB in conjunction with END. The average number of sentinel nodes harvested was three per patient. They found an overall NPV of 0.96 for SLNB, with no statistically significant difference between the various sites of the primary tumor. They did, however, find that the NPV was higher for T1 (NPV = 1) than for T2 (NPV = 0.94) tumors. They concluded that it may be reasonable to initiate clinical studies involving just SLNB and perform completion neck dissection only in patients with positive sentinel nodes.
A meta-analysis of 19 articles evaluating the effectiveness of SLNB in patients with head and neck SCC demonstrated an identification rate of 97.7% for sentinel nodes. The overall sensitivity was 92.6%, and analysis of the potential outcomes (e.g., recurrence, death, disease-free survival) for SLNB versus END showed the cumulative payoff for the SLNB group to be about 1% lower than that for the END group.
Drawbacks of this method include subjecting patients to a second operation for formal therapeutic neck dissection if the sentinel nodes are found to harbor tumor deposits on histopathologic review. Furthermore, the close proximity of some primary tumors (e.g., floor of the mouth) to the upper-echelon nodes can make it difficult to identify the sentinel nodes in this region, with scatter from the primary tumor obscuring readings of the nodes by the gamma probe. In addition, some of the nodes may be small and not readily accessible.
The use of SLNB can be a viable option for the management of cN0 necks in patients with early-stage SCC of the head and neck and may eventually prove to be the solution to the debate involving the close observation and elective treatment approaches. However, further studies are required to definitively demonstrate its clinical effectiveness, and a more standardized technique must be implemented among the various institutions.
Prognostic Variables for Nodal Metastasis
A retrospective cohort study of 105 patients with cN0 necks demonstrated that occult nodal metastasis occurred in 34% of the patients. Tumor thickness was the only independent predictor of nodal metastases, with thin (≤5 mm) and thick (>5 mm) tumors having a 10% and 46% risk, respectively, for the development of nodal metastases ( P = .001). Other variables, including the primary site, perineural invasion, lymphovascular invasion, histopathologic grading, and T stage, were not shown to independently predict nodal metastases on multivariate analysis. A thorough study by Yuen and colleagues evaluated multiple possible prognostic factors for nodal metastases in patients with oral tongue SCC and concluded that tumor thickness was the only independent predictor of nodal metastases. Similarly, Warburton and co-workers found that tumor thickness is the only statistically significant independent predictor for nodal metastases in oral cavity SCC.
Although tumor thickness has consistently been shown to be an independent risk factor for occult nodal metastasis, no uniform depth has been agreed on as the cutoff for prescribing elective neck treatment in patients with early-stage cancers, with the range of thickness in the literature being 1.5 to 8.0 mm. There are many possible explanations for this discrepancy, such as variability in the method of measuring tumor thickness, the specimen fixation method used, or inaccurate sampling of the tumor by incisional biopsy.
Radiation Versus Surgery
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


