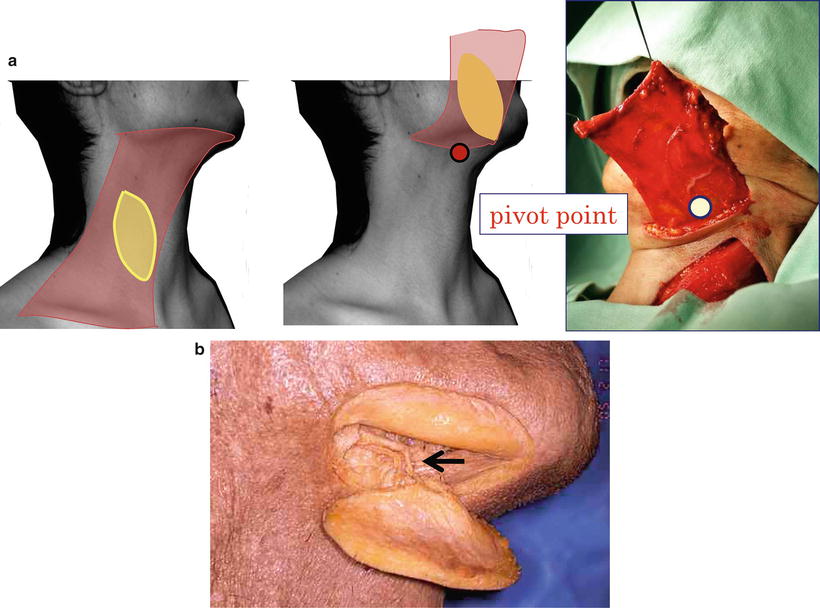
Fig. 9.1
Disability/rehabilitation medicine and maintenance of social life. (a) Tumor resection: maintenance of biological life. (b) Reconstruction and disability/rehabilitation medicine: maintenance of social life. (c) Oral cancer therapy today: treatment of the cancer itself and disability/rehabilitation medicine for any resulting impairment are essential, just like a set of wheels, and reconstructive surgery fills the role of an axle connecting these wheels
Impairment of function after extensive resection of oral cancer can be anticipated when “cancer” is diagnosed, surgery can be planned upon the occurrence of impairment [1, 2], and a rehabilitation plan can be designed before the impairment. These steps provide a clear advantage not available upon sudden onset of cerebrovascular disorders or accidents.
After surgical treatment of oral cancer, subsequent pre- and postoperative rehabilitation by specialists (otorhinolaryngology-head and neck surgeons, oral and maxillofacial surgeons, plastic and reconstructive surgeons, speech-language-hearing therapists, nurses, and clinical psychologists) is essential. It is therefore necessary for medical professionals to cooperate with one another for the benefit of the patients to whom they provide medical care and to facilitate their return to normal social life.
In cancer treatment, “treatment of the cancer itself” and “disability/rehabilitation medicine” for any resulting impairment are essential, just like a set of wheels, and reconstructive surgery fills the role of an axle connecting these wheels.
Reconstructive surgery is a science and an art. To achieve favorable outcomes, surgeons need to select transplantation procedures through which they can perform to their best and utilize their skills. Oral and maxillofacial reconstruction requires both functionality and aesthetics to which due consideration is requisite, despite the difficulties of the reconstruction.
9.1.2 Step-Surgery Concept
In oral and maxillofacial reconstructive surgery, there are two concepts regarding the principle of facial reconstruction methodology: (1) restoration of defects with facial and neck tissue, particularly with adjacent tissue if possible, and (2) unit principle, that is, reconstruction of each facial unit with color and texture. The former is feasible with regard to color and texture compatibility. The latter is subdivided from the aesthetic units [3] to sub- [4, 5] and mini-units [6]. Initial reconstruction with adjacent facial and neck tissue (applying the unit principle as needed) is conducive to acceptable outcomes for small facial defects. For moderate or more severe defects, however, the outcome of this initial reconstruction with adjacent tissue has often been unsatisfactory. Particularly, when the defect extends over 2 or more units or full thickness, reproducing the outline of each unit and its three-dimensional contour using the skin flap and graft is challenging because predicting and controlling three-dimensional changes induced by postoperative contracture and gravity is difficult. Moreover, when the optimum adjacent tissue for the reconstruction of the defect has already been used in the initial surgery, the most desirable outcome cannot, in many cases, be obtained by repeated revisions.
As a therapeutic policy for moderate or more severe full-thickness facial defects, we propose the step-surgery concept: (1) In the initial step, only filling and transplantation of minimum supporting tissue necessary for the restoration of function by free flap transplantation is carried out, and the surrounding tissue is conserved, (2) in the touch-up step, aesthetic surgery by transplantation of necessary supporting tissue is carried out with the use of a local facial/neck flap and skin graft, (3) as much cutaneous surface exposed on the face as possible is replaced with the facial/neck skin in the touch-up step, and (4) all the steps are planned as a series before surgery. Treatment based on this concept provides two advantages: (1) the free skin and musculocutaneous flaps transplanted in the initial step serve as a host side with abundant blood circulation, making secondary aesthetic surgery with a local skin flap easy, and (2) since the exposed cutaneous surface is resected after confirming time-course changes in the transplanted free flap, and since secondary surgery is carried out accordingly, the outline and contour are readily reproduced. When the cutaneous surface of the free flap is exposed on the face, differences of color match, texture, and thickness between the flap and the surrounding tissue present serious aesthetic problems; replacing the free flap with skin of the face/neck is desirable [7, 8]. When free flaps are used in reconstructive surgery, the importance of aesthetic outcomes should be taken into consideration.
The case presented here is of a 51-year-old man with recurrent squamous cell carcinoma of the left buccal mucosa after external irradiation (65 Gy). Resection of the full-thickness cheek, oral commissure, and vermilion was carried out. The number of defective units was 3, and there was no supporting tissue. The defects of full thickness of the cheek, upper lip, and oral commissure were reconstructed with free forearm and vermilion advancement flaps in the initial step, and aesthetic revision with a malar flap and a skin graft was carried out in the touch-up step. The outcome was acceptable. For patients who receive ≥50-Gy of irradiation, as in the present case, the initial step with the use of free skin or musculocutaneous flaps is very important (Fig. 9.2).
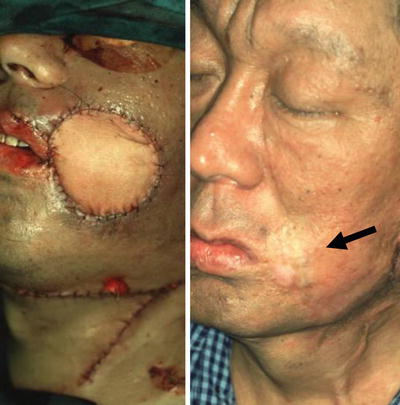

Fig. 9.2
Step-surgery concept. A 51-year-old man with recurrent squamous cell carcinoma of the left buccal mucosa after external irradiation (65 Gy). The defects of full thickness of the cheek, upper lip, and oral commissure (the number of defective units was 3, and there was no supporting tissue) were reconstructed with free forearm and vermilion advancement flaps in the initial step, and aesthetic revision with a malar flap and a skin graft was carried out in the touch-up step. For patients who receive ≥50 Gy of irradiation, as in the present case, the initial step with the use of free skin and musculocutaneous flaps is very important
9.2 Practice of and Strategy for Oral and Maxillofacial Reconstruction
9.2.1 Free Radial Forearm Flap
9.2.1.1 Role of Perforating Vein in Vascular Pedicle
The vascularized free forearm flap reported by Yang et al. [9] is now recognized as one of the most useful means for reconstructive surgery. Because of its long length, the large diameter of the blood vessel, and the flexibility of the cutaneous portion, it is widely used, particularly for head and neck, oral, and maxillofacial reconstruction [10–12]. The radial artery is generally used as the feeding artery. Two options for the drainage vein are the cephalic vein and radial comitant vein [9, 13]. The cephalic vein is generally selected because comitant veins in the forearm are very thin and not necessarily useful for vascular anastomosis. We have focused on the perforating vein connecting the deep venous and cutaneous venous systems since 1987 and have elevated it included in the vascular pedicle of forearm flaps. The perforating vein is situated anterior to the cubital fossa and is described in the first edition of Grant’s Atlas of Anatomy (1943). It branches from the radial comitant vein at a site slightly distal to the bifurcation of the radial and ulnar arteries and joins the medial cubital or radial cephalic vein (Fig. 9.3). The deep venous system comprises the radial comitant vein and comitant vein of the proximal brachial artery. The cutaneous venous system comprises the cephalic, medial cubital, and basilic veins. Thus, the perforating vein connects the deep and cutaneous venous systems (Fig. 9.4).
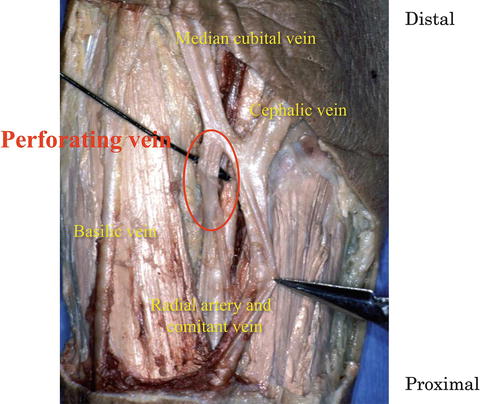
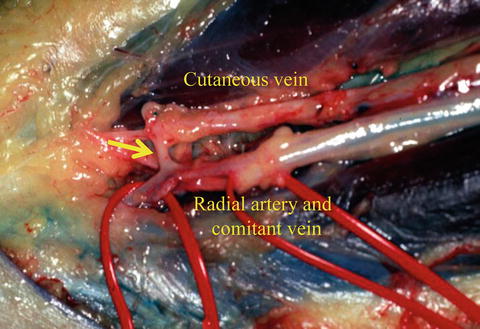

Fig. 9.3
Perforating vein. The perforating vein communicates between the radial comitant and cutaneous venous system

Fig. 9.4
Vascular pedicle of the forearm flap. The arrow indicates the perforating vein communicates between two venous systems
In conventional elevation of the forearm flap, only one of the two drainage vein systems is used—the cutaneous or deep venous system. By including the perforating vein in the vascular pedicle, however, both systems can be used for drainage through single venous anastomosis. Soutar et al. [11] have pointed out that the two venous systems of the forearm are connected through a vein flowing into the medial cubital vein. In this context, Evans et al. [14] have also pointed out that when the vascular pedicle is dissected at a site more proximal than at the perforating vein, circulation of the two systems is achieved by a single venous anastomosis. Timmons et al. [15] also have described the anatomical presence of a vein with a large diameter connecting the two venous systems. Thoma et al. [16] have demonstrated that the vena comitans generally joins the cephalic vein through another vein with a large diameter at a site anterior to the cubital fossa: a venous drainage pattern connecting the two venous systems through this other vein has been shown in 65 % of head and neck reconstruction cases with forearm flaps. These anastomosed veins are identical to the perforating vein described by us.
The usefulness of the perforating vein is summarized below. Venous drainage through the two systems is possible by anastomosis of either the cutaneous or deep vein. We have confirmed the absence of a venous valve in the perforating vein and its surroundings. Therefore, blood flow in the perforating vein may differ between cutaneous and deep vein anastomosis (Fig. 9.5). This corresponds to the “oscillating vein” proposed by Taylar et al. [17]. Timmons et al. [15] have demonstrated anatomically that, generally, the direction of venous valves in the comitant vein is determined so as to maintain the direction of blood flow from the deep to the cutaneous venous system, suggesting that this is the standard direction. Accordingly, inclusion of the comitant vein in the drainage system may provide a more advantageous circulatory condition, particularly for initial drainage after reperfusion. We have demonstrated this experimentally [18, 19]. Even when only the cutaneous vein is anastomosed, the perforating vein is capable of maintaining drainage through the comitant vein and reducing congestion of the flap in the initial phase after reperfusion, compared with drainage through only the cutaneous vein. Even when a flap is elevated with the radial artery and vein, excluding the cutaneous vein in the distal forearm, the cutaneous vein with a large diameter in the cubital fossa can be used for vascular anastomosis due to the connection through the perforating vein (Fig. 9.6). For example, women with very thin cutaneous veins difficult to identify and patients with unusable forearm cutaneous veins because of chemotherapy are good candidates for this method.
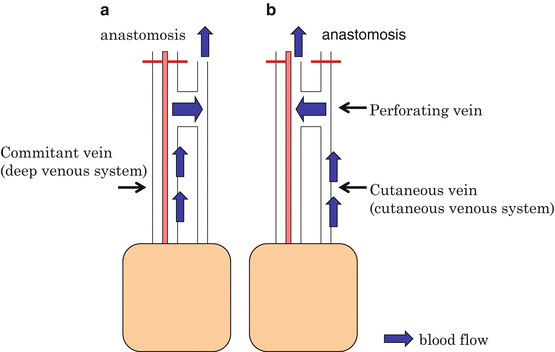
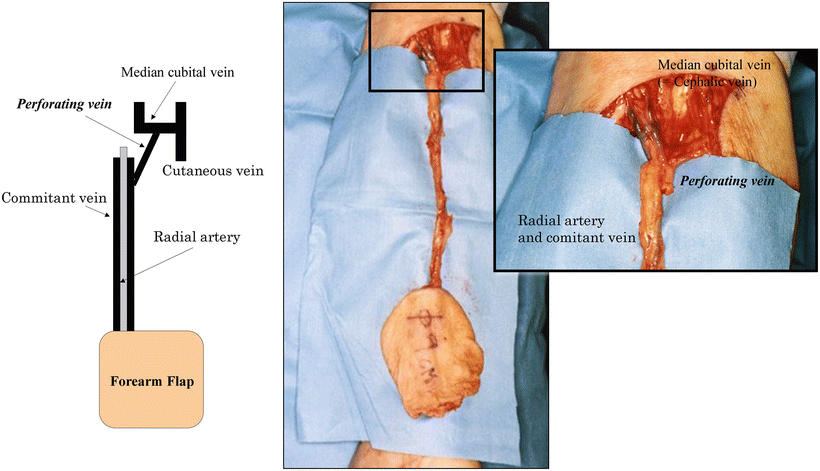

Fig. 9.5
Perforating vein as “oscillating vein.” Venous drainage through the two systems is possible by anastomosis of either the cutaneous and deep vein. The absence of venous valve in the perforating vein and its surroundings has been confirmed. The blood flow of the perforating vein may differ between cutaneous and deep vein anastomosis. This corresponds to the “oscillating vein”

Fig. 9.6
Usefulness of the perforating vein. Even when only the cutaneous vein is anastomosed, the perforating vein is capable of maintaining drainage through the comitant vein (deep venous system). Even when a flap is elevated with the radial artery and vein, excluding the cutaneous vein in the distal forearm, the cutaneous vein with a large diameter in the cubital fossa can be used for vascular anastomosis due to the connection through the perforating vein
The drawbacks of this method are: (1) vascular ligation up to the brachial artery and vein is complex, (2) ligation and division of a vein with a large diameter in the cubital fossa may cause edema [16], and (3) a slightly long scar is unavoidable because the skin incision is extended to the cubital fossa.
9.2.1.2 Simple Dressing Technique Using Polyurethane Foam for Fixture of Skin Grafts
Various methods of grafting skin, including tie-over dressing, are used depending on the location, area, and shape of the graft [20–23]. We use polyurethane foam (Allevyn Hydrocellular Dressing® (AHD®), Smith & Nephew, Largo, FL) and polyurethane film (Tegaderm®, 3M, St Paul, MN) to affix a skin graft to the flap-donor region of the forearm. The procedure comprises four steps: (1), a skin graft is sutured to the wound remaining elevating a forearm flap, and the donor bed is washed well with physiological saline, and a small hole for drainage is made or a quilted suture is used, as needed (Fig. 9.7a), (2) a sheet of polyurethane foam (AHD)® of almost the same size as that of the skin graft is placed over the skin graft and covered with another sheet about 1–2 cm wider (Fig. 9.7b), (3) the two AHD® sheets are affixed with adhesive polyurethane film (Tegaderm®), and (4) the procedure is completed with bandage.
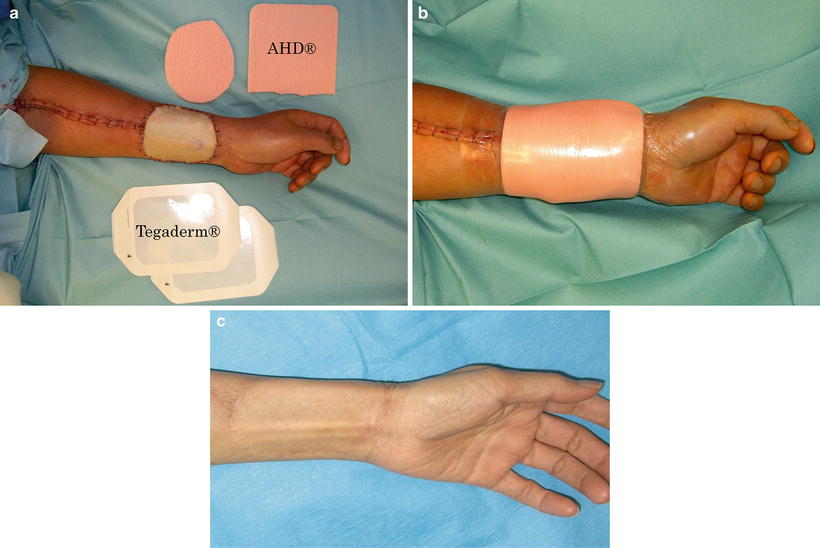

Fig. 9.7
Surgical procedure using polyurethane foam for fixture of skin graft. (a) The skin graft is fixed with 5-0 nylon suture and two sheets of polyurethane foam (AHD®) are placed on the skin graft: the first is one of the same size as the graft, and the second is one size larger to overlap by 1–2 cm the edges of the first sheet. (b) The ADHs are fixed with adhesive polyurethane film (Tegaderm®). Dressing is simple and easy without an assistant. The same procedure can be repeated at any time. (c) The region of the skin graft heals flawlessly, under a moist healing condition, and the postoperative treatment period is short
The duration of fixture is the same as that for tie-over dressing, but this is not strictly specified because the fixture can be readily repeated at bedside. When grafting skin to the forearm, heed should be paid to the formation of hematoma and to partial necrosis attributed to overpressure [24, 25]. Favorable grafts are obtained by fixtures at about 10 mmHg [26]. The surface of the wound on the forearm flap-donor region becomes uneven because of exposure of the tendon. Application of even pressure onto the wound surface is difficult, and the conventional method may exert overpressure on convex regions. In our method, elastic AHD exerts appropriate pressure on the graft. Also, since two AHD sheets are tiered, the lower sheet fits the morphology of the skin defect and rules out dead space; the slightly larger upper sheet prevents detachment of the skin graft, and the polyurethane film (Tegaderm®) fortifies the fixture. The region of the skin graft heals flawlessly under a moist healing condition, and the postoperative treatment period is short (Fig. 9.7c).
9.2.1.3 Reflex Sympathetic Dystrophy After Free Radial Forearm Flap Elevation
Reflex sympathetic dystrophy (RSD) is an intractable chronic pain syndrome whereby persistent pain, edema, and abnormal sweating develop after trauma and surgery, mainly in the injured region, and the tissue finally atrophies [27]. Clinical symptoms are observed over the entire upper limb of the forearm flap-donor region—a complication after elevating a forearm flap—to which due attention should be paid.
In the patient presented here, numbness and spontaneous pain developed mainly in the flap-donor region about 3 months after surgery (Fig. 9.8a, b). The range of symptoms gradually increased, and skin redness and edema appeared throughout the upper left limb about 6 months after surgery. One year after surgery, the patient developed a severe burning sensation throughout the limb induced by even a slight touch of clothing in the area, and then pain began to radiate to the left shoulder (Fig. 9.8c). Diagnosed with RSD at the Department of Anesthesia, the patient was treated with stellate ganglion block with 6 ml of 1 % lidocaine 3–4 times a month at a pain clinic. Skin redness and edema of the forearm remitted after 20 cycles of the treatment and were mostly resolved after 40 cycles (about 1 year)
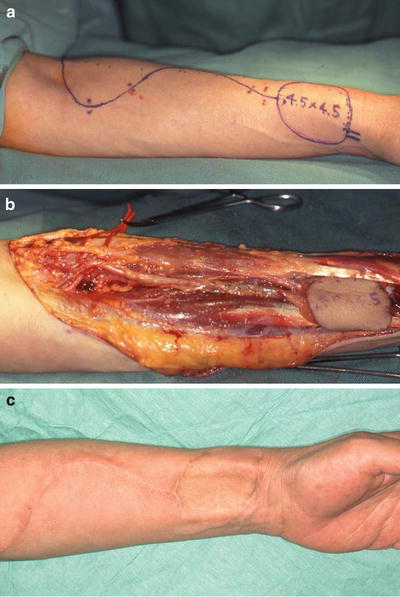

Fig. 9.8
A case of RSD after forearm flap elevation. (a) Design of forearm flap. (b) Elevation of forearm flap: lateral antebrachial cutaneous nerve and superficial branch of the radial nerve were resected in this case. (c) Severe burning sensation and tenderness were found at the left forearm, which rapidly spread to the upper limb after surgery
In artificial synapse formation, a theory of the developmental mechanism of RSD, a synapse is artificially formed between nerve fibers in an injured peripheral nerve region, and sympathetic distal impulses are transmitted as pain to pain-transmitting fibers [28]. In this patient, an artificial synapse may have formed between the lateral antebrachial cutaneous nerve and the superficial branch of the radial nerve in the injured region of the forearm, inducing pain. Clinical diagnosis of RSD is based on a score established by Gibbons et al. [29] (Table 9.1). The patient’s score was 5 based on the symptoms of pain hypersensitivity, burning sensation, skin color tone, and fluctuations in skin temperature, leading to our diagnosis of RSD. The principle of the management of RSD is early diagnosis and treatment, especially that progression to the chronic phase makes recovery difficult by any method of treatment. For RSD of the upper limbs, stellate ganglion block is the most effective. Edema and color change associated with the sympathetic nerve may be improved by stellate ganglion block, but peripheral nerve block has in some studies been described as more effective.
Table 9.1
RSD score
|
1. Allodynia or hyperpathia
|
1.0
|
|
2. Burning pain
|
1.0
|
|
3. Edema
|
0.5
|
|
4. Color or hair growth change
|
1.0
|
|
5. Sweating change
|
0.5
|
|
6. Temperature change
|
0.5
|
|
7. Radiographic change (demineralization)
|
0
|
|
8. Quantitative measurement of vasomotor/sudomotor disturbance
|
0
|
|
9. Bone scan consistent with RSD
|
0
|
|
10. Response to sympathetic block
|
0.5
|
9.2.2 Vascularized Free Rectus Abdominis Musculocutaneous Flap in the Oromandibular Region in Terms of Efficiency of the Anterior Rectus Sheath
The anterior rectus sheath is one of the firm lateral abdominal aponeuroses of the abdominal external oblique muscle. The efficiency of the sheath in reconstructive surgery has been demonstrated in the repair of full-thickness right ventricular defects after mediastinitis or sternal dehiscence [30], in the treatment of bronchopleural fistulas complicated by empyema [31], in pleural reconstruction of the chest wall [32], dural reconstruction [33], and breast reconstruction after subcutaneous mastectomy [34]. We have utilized this sheath in oromandibular reconstruction with the vascularized free rectus abdominis musculocutaneous flap (RAM). The RAM is effective in the maintenance of the formed bulge of the oral floor, prevention of the sinking of the reconstructed tongue, and prevention of the exposure of reconstruction plates after the resection of the mandibular continuity. Therefore, we consider that RAMs are absolutely indicated for these types of reconstruction (Table 9.2). As is important, the muscle attached to the rectus sheath is harvested with a 3- to 4-cm extension superiorly and inferiorly (Fig. 9.9). Extensive resection of the anterior rectus sheath caused no problems.
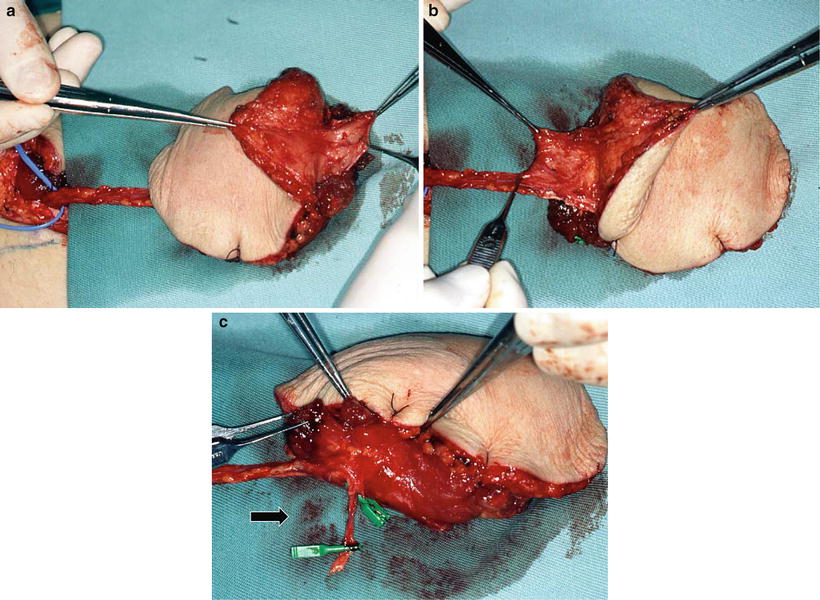
Table 9.2
Purpose and devices of reconstruction with RAM
|
Resection procedure of primary tumor
|
Purpose of reconstruction
|
Device
|
|---|---|---|
|
Resection of the oral floor with hemiglossectomy
|
Preservation of swallowing
|
Hammock technique a
|
|
Total resection of the mobile tongue or more extensive resection
|
Preservation of swallowing and articulation
|
Money-pouch-like reconstructionb + hammock technique + cricopharyngeal myotomy + laryngeal suspensionc + neuroanastomosisd
|
|
Resection of the mandibular continuity with oral defect
|
Prevention of reconstruction plate exposure
|
Reinforcement of the muscular portion
|

Fig. 9.9
Preparation of anterior rectus sheath and tenth intercostal nerve. Raising the RAM is designed so that the center of the flap is positioned at the paraumbilical region. The rectus sheath is obtained with proximal and distal extensions of about 3–4 cm each, which is particularly important to attain the final goal of reconstruction with the sheath (a, b). When the intercostal nerve is used in reconstruction, the tenth intercostal nerve as a motor nerve of the lower abdominal rectus abdominis muscle is carefully distinguished from the paraumbilical region (arrow: the tenth intercostal nerve) (c)
In the preparatory phase of swallowing, the tongue margin and the oral floor elevate, retaining the alimentary bolus in the oral cavity with glossopalatal closing function [35] (Fig. 9.10). In reconstructive flaps, however, this function is impaired: the bolus on the reconstructed oral floor cannot be moved to the dorsum of the tongue. When the oral floor is reconstructed with a thin flap, as with a forearm flap to reform it to the pre-resection state, a wide depression results, where saliva and food residues become awkwardly trapped. This makes smooth food transfer difficult, causing a time lag between glossopalatal closure and bolus transfer, as well as mistimed swallowing as a whole resulting in misswallowing [36]. For an efficient swallowing function, it is essential to raise the oral floor to a height similar to that of the tongue margin, thereby forming the preparatory phase of swallowing statically. In other words, the nonfunctioning oral floor should not be depressed. Even when the bulge of the floor is formed with thick and bulky reconstructive materials, like the musculocutaneous flap, the cutaneous and muscular portion, in time, generally sags because of gravity, resulting in the depression of the oral floor. Therefore, we developed a method of forming the mylohyoid muscle-like tissue by fixing the anterior rectus sheath anteriorly and posteriorly to the mandibular areas in a hammock pattern. This protects the muscle from the influence of gravity, allowing the maintenance of the bulge and preventing the sinking of the reconstructed oral floor and tongue. We have termed this method the hammock technique, where a musculocutaneous flap with a firm anterior sheath is indispensable and where RAM is the optimal material. Although the mylohyoid muscle attaches to the hyoid bone, and elevates the oral floor and hyoid bone, in reconstructive surgery, the anterior sheath is not fixed at the hyoid bone because recovering the function by elevating the reconstructed oral floor is not possible even if such fixation is done. Conversely, fixation to the hyoid bone results in flap pulling and, consequently, in hindering the formation of the bulge of the reconstructed oral floor and the raising of the reconstructed tongue by the money-pouch-like reconstruction [37] (Figs. 9.11 and 9.12)
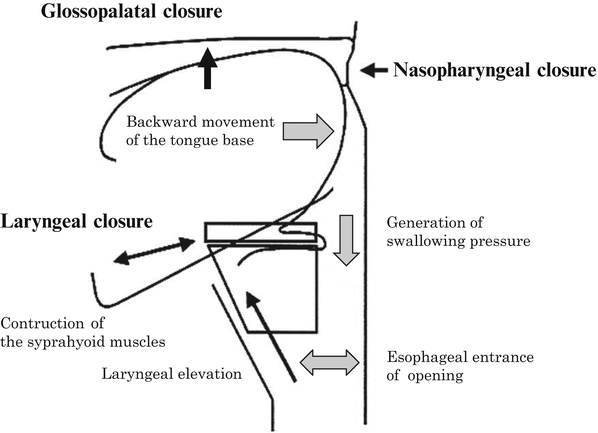
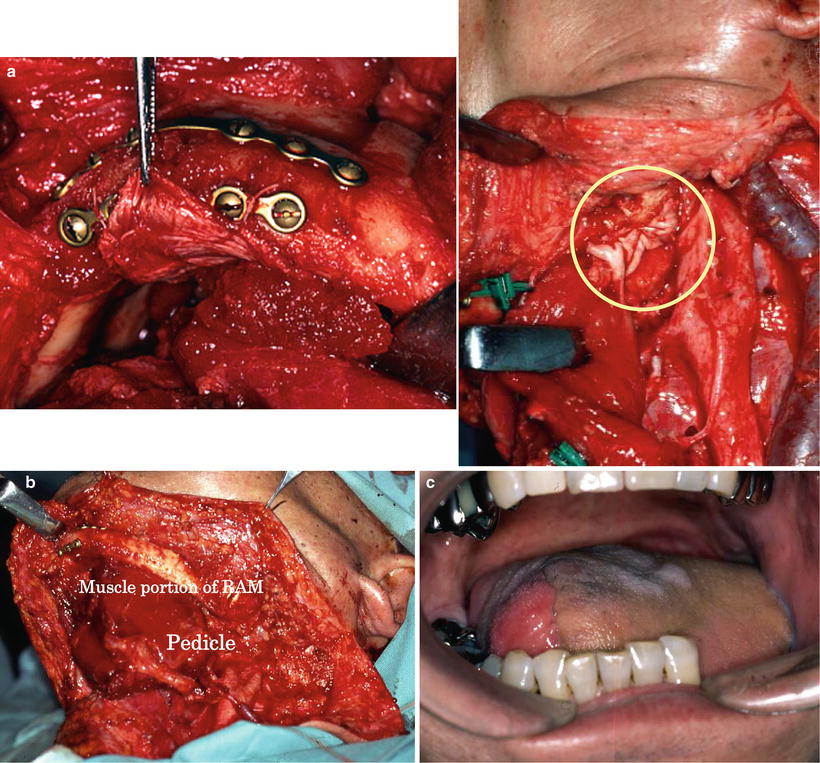
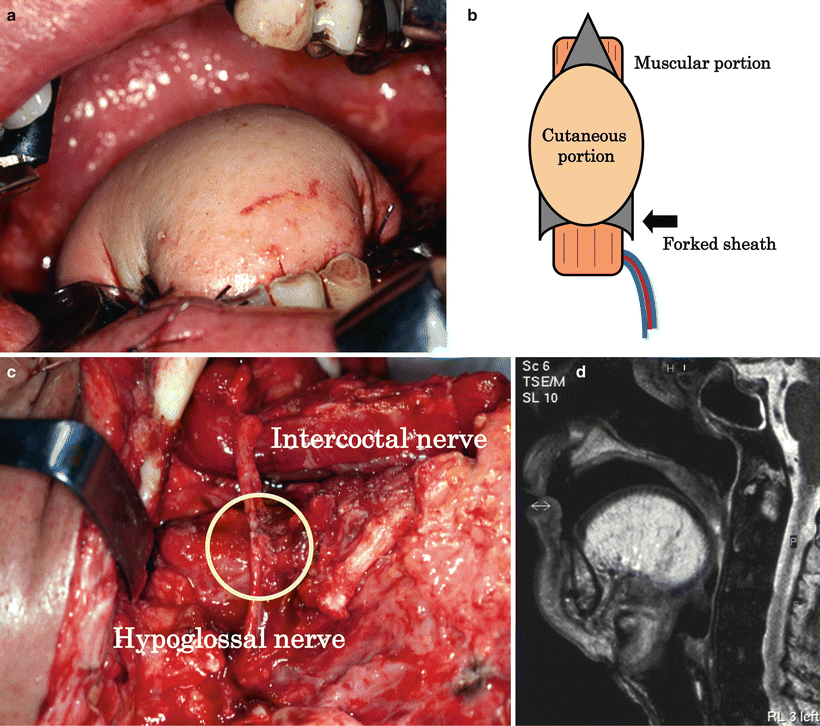

Fig. 9.10
Ideal static reconstruction of the oral and the pharyngeal phase of swallowing

Fig. 9.11
Reconstruction after resection of oral floor with hemiglossectomy. A 67-year-old man with squamous cell carcinoma of the left oral floor (T3N1M0) underwent resection of the oral floor with hemiglossectomy and right radical neck dissection. Hammock technique: the anterior rectus sheath was fixed anteriorly and posteriorly to the mandible and hung in a hammock pattern (a, round: mandibular angle), forming a mylohyoid muscle-like structure (b). Simultaneously, the oral floor was reconstructed to form a bulge. Seven years after the operation, the bulge of the reconstructed oral floor was maintained without gravity-induced depression (c)

Fig. 9.12
Reconstruction after total glossectomy. A 51-year-old man with advanced squamous cell carcinoma of the right tongue (T3N2cM0) underwent total glossectomy and bilateral radical neck dissection. Money-pouch-like reconstruction: the reconstructed tongue was rounded and markedly raised by the money-pouch-like reconstruction method. This technique gave the reconstructed tongue glossopalatal closing, and the mobility of the tongue base was preserved (a). Preparation of the proximal anterior rectus sheath: the anterior rectus sheath was fixed anteriorly and posteriorly to the mandible, in a hammock pattern, to form a mylohyoid muscle-like structure and to prevent flap sinking. Since the posterior area is sutured to the bilateral sides of the molar areas, the sheath should be prepared in a forked shape (b). Neuroanastomosis: the intercostal nerve is anastomosed to the hypoglossal nerve to reinnervate the rectus abdominis muscle and to prevent fatty degeneration and atrophy (round: neuroanastomosis) (c). MRI after 6 months showing the absence of flap sinking of fatty degeneration and of atrophy of the rectus abdominis muscle (d)
After total resection of the mobile tongue or more extensive resection, it is essential for the purpose of generating swallowing pressure to build the reconstructed tongue with height and roundness, to make the oropharyngeal space narrow, to provide the glossopalatal closing function, to maintain the mobility of the tongue base, and to avoid forming a gap to the posterior pharyngeal wall (Fig. 9.10). To achieve this outcome, the following techniques are essential: (1) the hammock technique (the bulge of the reconstructed oral floor is maintained, and the depression of the reconstructed tongue is prevented), (2) the money-pouch-like reconstruction method [37] (the tongue is reconstructed to give it height and roundness), (3) neuroanastomosis [35] [38] (the reinnervated muscle is used to maintain the bulk of the muscular portion of the RAM), (4) cricopharyngeal myotomy, and (5) resection of the infrahyoid muscles and thyroid cartilage/hyoid bone/mandible fixation. With these serial reconstruction procedures, the anatomical and functional structures of swallowing from the preparatory phase to the pharyngeal phase are statically reconstructed. Therefore, the residual tissues easily assist in the swallowing function.
In reconstruction after the resection of the mandibular continuity with oral defects, vascularized free osteocutaneous flaps are usually the first choice. When bone reconstruction is not possible because of insufficient conditions, however, mandibular continuity is restored with a reconstruction plate. The muscular portion and the anterior sheath are wrapped around the plate, whereby the anterior rectus sheath firmly reinforces the muscular portion. The flap margin is deepithelialized and submucosally inserted into the oral cavity; consequently the sheath on the muscular portion is further reinforced by the dermis. We term this method the wrap-around technique for mandibular reconstruction. To date, plate exposure has not been observed in any of the patients who underwent the procedures of this technique with the anterior rectus sheath (Fig. 9.13). After primary reconstructive surgery, usually 6 months thereafter, secondary generative mandibular reconstruction was carried out by implanting titanium mesh and particulate cancellous bone and marrow (PCBM) in the mandibular defect. A 3D model was prepared from CT images, and the mandibular morphology on the healthy side was reproduced in the defective side using the titanium mesh as a scaffold. PCBM collected from the posterior iliac crest was then buried in the rectus abdominis muscle. New bone formation was usually confirmed 3 months after the surgery, and regenerative mandibular reconstruction was completed in the next 6 months (Fig. 9.14).
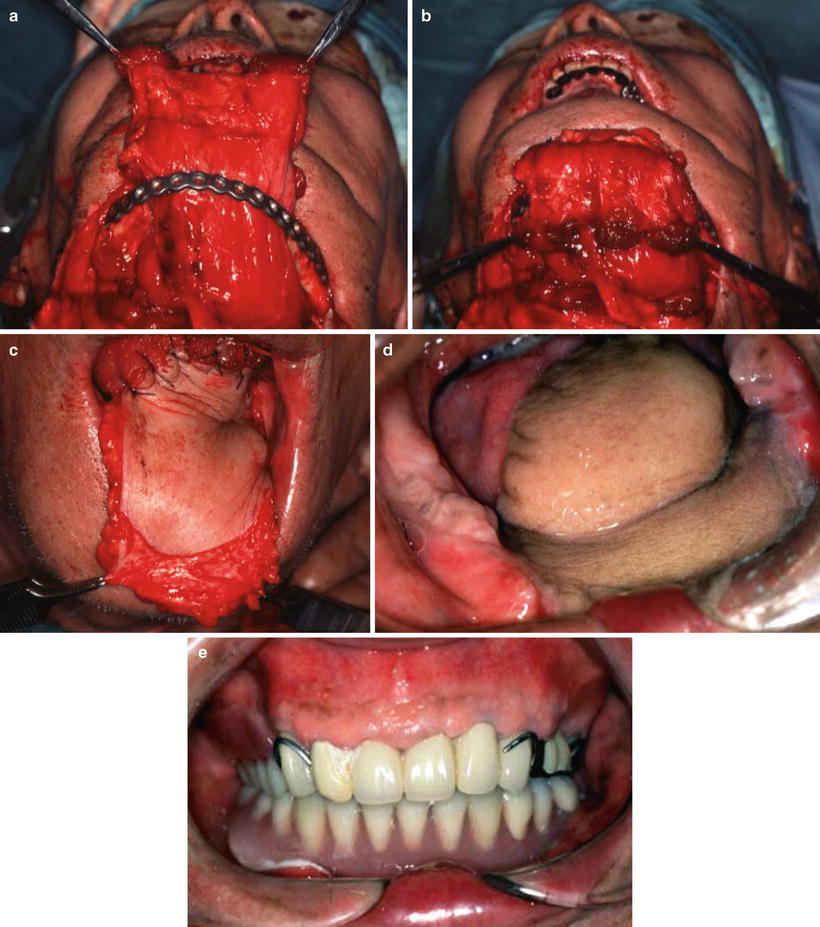
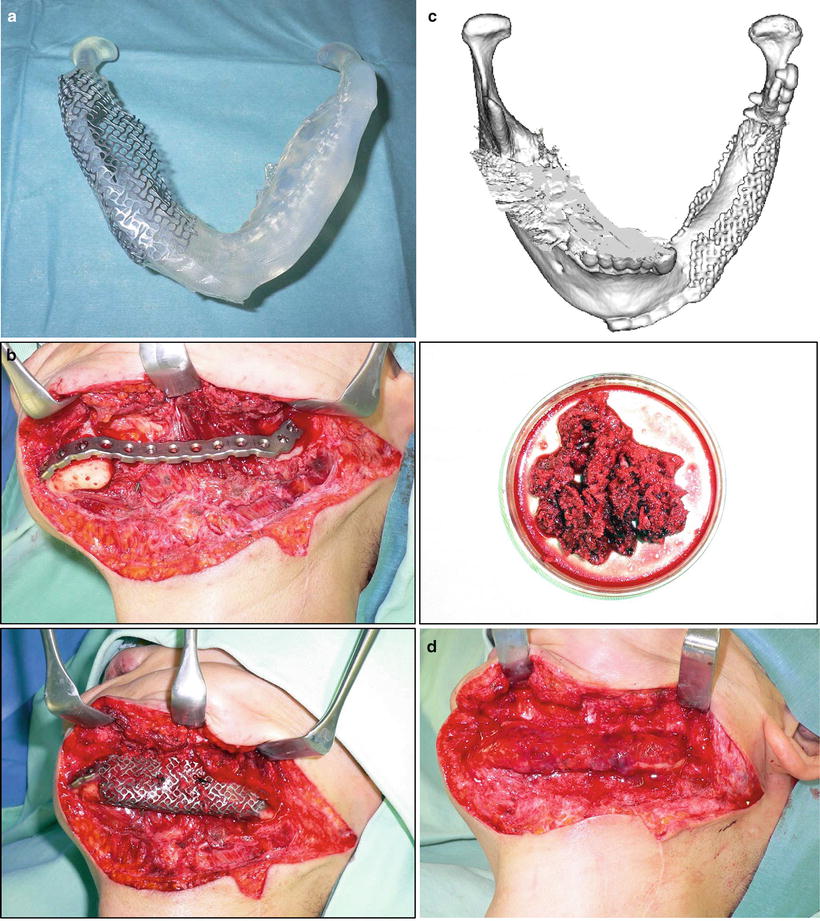

Fig. 9.13
The wrap-around technique for mandibular reconstruction. A 65-year-old man with squamous cell carcinoma of the left oral floor (T4N2bM0) underwent resection of the tumor, anterior hemoglossectomy, resection of the anterior mandibulectomy, and left radial and right supraomohyoid neck dissection. The muscular portion with the anterior rectus sheath was wrapped around the reconstruction plate. The anterior rectus sheath firmly reinforced the muscular portion (a, b). The flap margin was deepithelialized and submucosally inserted into the oral cavity, by which the sheath on the muscular portion was further reinforced by the dermis (c). No plate exposure was observed 4 years after operation (d). After mandibular denture application, masticatory function recovered (e)

Fig. 9.14
Regenerative mandibular reconstruction. (a) A 3D model was prepared from CT images, and the mandibular morphology on the healthy side was reproduced in the defective side using the titanium mesh as a scaffold. (b) PCBM collected from the posterior iliac crest was buried in the rectus abdominis muscle. (c) New bone formation was confirmed 3 months after surgery. (d) Regenerative mandibular reconstruction was completed in the next 6 months
Alternative to the muscle sheath alone, the tensor fascia lata flap may also serve as material for these reconstructions [39]; however, RAM appears to be the most efficacious in terms of the facility of flap elevation, the wide caliber and long length of the pedicle, the flexibility of the cutaneous portion (especially, the application to three-dimensional reconstruction), and the maintenance of the bulk of the muscular portion by reinnervation.
9.2.3 Double Pedicled (DOP) and Supercharged (SUP) Pectoralis Major Musculocutaneous Flap
Although free tissue transfer is the preferred reconstruction option in most major oral and maxillofacial reconstructions, the pectoralis major musculocutaneous (PMMC) flap is commonly used in the salvage of necrotic free flaps and is the first choice for patients who are not candidates for free flaps. The PMMC flap has a high incidence of distal skin necrosis, however, because of vascular insufficiency resulting in partial to total flap loss and fistula formation [40]. The pectoral branch of the thoracoacromial artery is the main blood supply to the skin island overlaying the upper part of the pectoralis major muscle. The lateral thoracic artery and the anterior intercostals branches of the internal mammary artery supply the skin region overlaying the lower part of the pectoralis major muscle [41–43]. The skin island must be designed in the lower chest to attain a pedicle length sufficient for oral and maxillofacial reconstruction. In the conventional harvesting method for oral and maxillofacial reconstruction, the lateral thoracic artery and all intercostal branches from the internal mammary vessel are cut to avoid compromise of arc of rotation of the flap. The dissections of these two dominant sources of blood supply to the skin island overlaying the lower PMMC flap poses a high risk of distal flap necrosis. The PMMC flap preserves the lateral thoracic vessels in addition to the pectoral branches of the thoracoacromial artery and is, therefore, a very valid choice from the viewpoint of blood supply [41, 44] (Fig. 9.15). Cadaver dissection has also shown that the lateral thoracic artery is a more dominant pedicle of the PMMC flap than the pectoral branch of the thoracoacromial artery in approximately 6 % of all cases studied. This percentage was very close to the total rate of skin necrosis of PMMC flaps [45]. Dissecting the lateral thoracic artery as a routine harvesting technique in such cases could result in total loss of the pectoralis major skin flap.
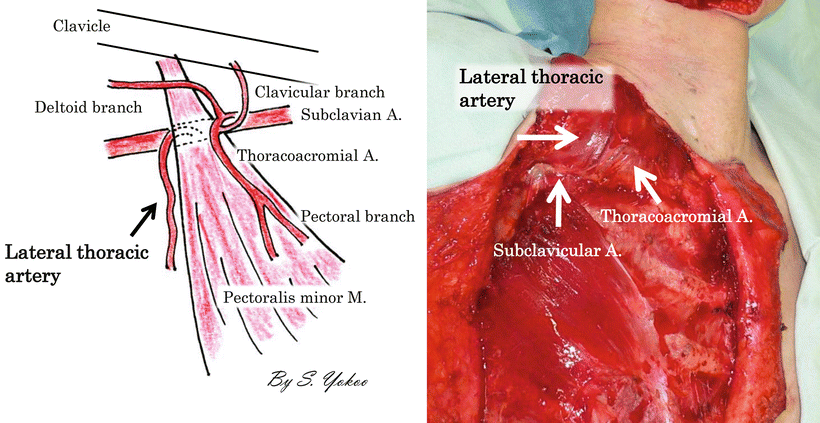

Fig. 9.15
Vascular anatomy of pectoralis major muscle. The thoracoacromial axis classically divides into four main branches: the clavicular, deltoid, pectoral, and acromial arteries. The lateral thoracic artery may also arise from this system, but, more commonly, branches out separately from the axillary artery. The thoracoacromial artery commonly divides into two major branches: the pectoral and deltoid. The acromial and clavicular arteries variably arise from either division. The deltoid arteries run in the deltopectoral groove with the cephalic vein, supplying both the pectoralis major and deltoid. It gives off a cutaneous perforator in the midpoint of the deltopectoral groove. The acromial branch contributes to a vascular plexus along with branches from the deltoid, suprascapular, and posterior humeral circumflex vessels. The clavicular branch runs a cephalad and medial course toward the sternoclavicular joint. The pectoral branch pieces the clavipectoral fascia and then runs a cephalocaudal course on the deep surface of the pectoralis major muscle, which it supplies
A skin island designed in the lower chest to reach the oral and maxillofacial defect includes the fourth intercostal perforating branches [43]. The muscle is elevated from the chest wall inferiorly to superiorly. The pectoral branch of the thoracoacromial artery is included in the flap as in the conventional harvesting method of the PMMC flap. The lateral thoracic artery, identified underneath the lateral border of the pectoralis muscle in the region of the maxilla, comes out underneath the lateral border of the pectoralis minor muscle and enters the lateral part of the pectoralis major muscle [46]. The pectoralis minor muscle overlying the lateral thoracic artery is dissected completely to release the lateral thoracic artery up to the clavicle [42]. The lateral thoracic vessels are kept pedicled, not cut, if the length of the pedicle is not compromised. This is called “double pedicled pectoralis major musculocutaneous flap” or “DOP-PMMC flap.” Lateral thoracic vessels should be preserved with the pedicle, not dissected, when the length of the pedicle is not compromised in oral and maxillofacial reconstruction; the flap could reach the oral cavity without limitation, when the pectoralis minor muscle is completely dissected. Compromise of pedicle length is often experienced, especially when wrapping the muscle of the PMMC flap around the titanium reconstruction plate used in segmental mandibulectomy (wrap-around procedure), even when the pectoralis minor muscle is completely dissected. The compromise may be due to the long distance between the thoracoacromial artery and the lateral thoracic at the bifurcation of the subclavian artery, to the short length of the lateral thoracic artery from the bifurcation of the subclavian artery to the point of entry into the pectoralis major muscle, or to the constitution of the patient—a long neck compared with the chest. In such cases, the lateral thoracic vessels are cut at the bifurcation of subclavian vessels, and then microvascular anastomosis is carried out between lateral thoracic vessels and the transverse cervical artery and external jugular vein. We named our new procedure “supercharged pectoralis major musculocutaneous flap (SUP-PMMC flap).” Thus, the lateral thoracic artery is preserved without any compromise of pedicle length, which takes into consideration the blood supply of the lower part of PMMC flaps. Lateral thoracic vessels are suitable for microvascular anastomosis as described for the free lateral thoracic flap and for the lateral thoracic perforator flap [47, 48]. It should be noted that a deficit of the lateral thoracic artery has been reported in approximately 15 % of cases [47, 49]. Although we have not been able to fully resolve the issue of compromised distal skin blood supply because of the necessity of dissecting the branches of the internal mammary artery, it is worthwhile to preserve the lateral thoracic vessel that is the major contribution to the lateral and distal PMMC flap (Fig. 9.16).
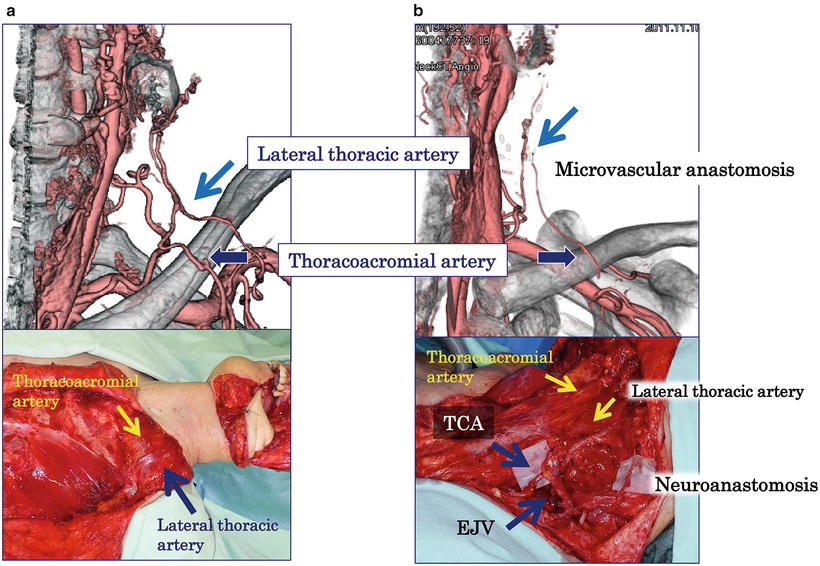

Fig. 9.16
Computer tomographic angiography of DOP- and SUP-PMMC flap. Computer tomographic angiography shows the lateral thoracic artery and pectoral branch of the thoracoacromial artery of DOP- (a) and SUP-PMMC flap (b) one year after surgery. The great auricular nerve is also anastomosed to the medial pectoral nerve to prevent muscle atrophy. TCA transverse cervical artery, EJV external jugular vein
9.2.4 Platysma Flap and Cervical Island Flap
The application of a cervical skin flap to oral and cervical defects, subject of many reports, was initially described as “the apron flap” by Ward et al. in 1950 [50]. In 1969, Farr et al. [51] have described a similar flap as a cervical island skin flap. In both studies, blood circulation is described as being of a random pattern. In 1978, Futrell et al. [52] have described an axial-pattern flap by using the submental branch of the facial artery, the dominant pedicle of the platysma, for feeding the platysma musculocutaneous flap and have used it in oral reconstruction. Although the platysma flap and cervical island flaps are very similar, their circulation systems are totally different and should be clearly differentiated, yet they are still being confused in many studies [53]. By taking the circulation systems into consideration, a platysma flap can be elevated safely.
Since the platysma flap is of an axial pattern that uses the submental branch of the facial artery as the dominant pedicle, the skin flap is a complete island. The suprasternal branch of the suprascapular artery and the platysma branch of the suprathyroid artery are minor pedicles; therefore, their circulation system is Mathes’s type II [54, 55]. When a skin flap is used for oral reconstruction, cutting the minor pedicle is requisite, and the skin flap is fed only by the dominant pedicle present in the upper region. Thus, the arc of rotation is determined by this artery (Fig. 9.17). The anterior region in neck skin receives blood supply from the submental branch of the facial artery, with the anterior margin of the sternocleidomastoid muscle regarded as the boundary [56]. Accordingly, a skin island of the platysma flap should be set in this region or in a region from where subcutaneous (dermal) blood flow is foreseen (Fig. 9.18). The submental branch of the facial artery and its arborization are distributed in the deep adipofascial layer and extend perforators to the platysma and skin. Thus, no axial-pattern circulation is obtainable unless this layer is conserved. Imanishi et al. [57] have shown that the dominant pedicle extends a long branch to the deep adipofascial layer and forms a vascular plexus, but it extends only a small vessel to the platysma, and no vascular plexus is formed between the platysma and skin. Thus, elevating a platysma flap as a musculocutaneous flap is risky; it should be elevated as an adipofasciomusculocutaneous flap. This anatomical fact is very important when elevating a platysma flap (Fig. 9.19). Venous circulation in the platysma flap is retrogradely drained by including the external jugular vein [56]. The deep adipofascial layer into which the jugular vein distributes is important for venous circulation. Skin loss has been reported in 20–70 % of platysma flaps [58], but has occurred in only 3 % with the use of our method of elevation. From the viewpoint of cervical lymph flow based on Harnsberger’s fascia classification [59], a platysma flap destroys the structure of the cervical fascia. Moreover, since the deep adipofascial layer is present in the superficial space [59], the platysma flap includes the superficial cervical lymph node. Taken together, these conditions may induce a late neck lymph node metastasis-like abnormality. Nonetheless, we have not encountered any late metastatic case assumed to have induced an abnormality in cervical lymph flow (Fig. 9.20).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses