Introduction
The aim of this study was to determine the peri-miniscrew implant crevicular fluid receptor activator of nuclear factor-кB ligand (RANKL) and osteoprotegerin (OPG) levels around loaded and unloaded miniscrew implants at different time intervals.
Methods
Twenty loaded and 16 unloaded miniscrew implants were included in this study. All miniscrew implants were placed bilaterally between the maxillary second premolars and first molars as anchorage units for canine distalization. Peri-miniscrew implant crevicular fluid was taken from the mesiobuccal aspects of the loaded and unloaded miniscrew implants before loading; at 24, 48, and 168 hours; and on day 30 after force application. Enzyme-linked immunosorbent assay kits were used to determine RANKL and OPG levels in the peri-miniscrew implant crevicular fluid samples. Wilcoxon, Mann-Whitney U, and Spearman correlation tests were used for statistical evaluations at the P <0.05 level.
Results
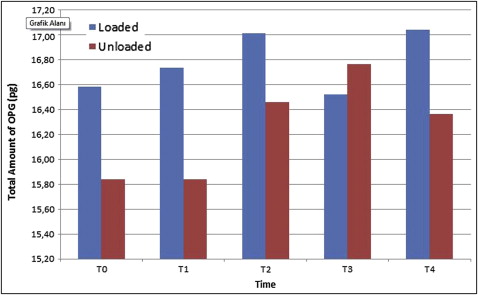
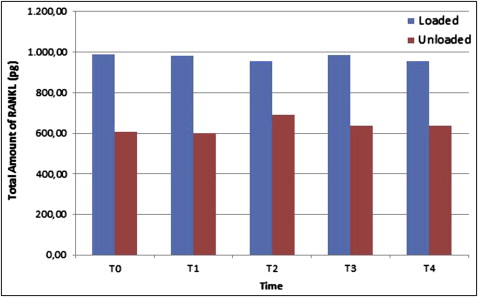
Although the total amount of OPG was not different between the groups, the total amount of RANKL was significantly elevated in the loaded miniscrew implant group ( P <0.05) at all time periods. Peri-miniscrew implant crevicular fluid volume was the highest at 48 hours in the loaded group. Also, the OPG/RANKL ratio in the peri-miniscrew implant crevicular fluid was significantly decreased in the loaded miniscrew implant group.
Conclusions
The OPG and RANKL levels vary around loaded and unloaded miniscrew implants as a result of force application.
Skeletal anchorage with dental implants, miniplates, miniscrews, and microscrews provides absolute anchorage for tooth movement. Miniscrew implants have many benefits, such as immediate or early loading, ease of placement and removal, and relatively low cost. These advantages have expanded the use of miniscrew implants for various orthodontic treatments. Both clinical and experimental studies have demonstrated that miniscrew implants can provide sufficient and stable anchorage for tooth movement during the entire orthodontic therapy.
Mechanical stimulation can initiate and promote bone remodeling. Osteoblasts and osteoclasts are specialized cells responsible for bone formation and resorption, respectively. Many investigators have studied the interaction between these 2 cell types. Recently, osteoprotegerin (OPG) and receptor activator of nuclear factor-кB ligand (RANKL) have been shown to play important roles in bone remodeling. OPG is a member of the tumor necrosis factor receptor family and a soluble decoy receptor against RANKL. It is produced by osteoblasts and other cells and has been found to be a key factor in the inhibition of osteoclast differentiation and activation. In contrast, RANKL, which is expressed on the surface of osteoblasts, activates differentiation of preosteoclastic cells into mature osteoclasts and thus promotes bone resorption. The activation of clastic cells is regulated by the activation of the receptor activator of NF-kB. RANKL binds to receptor activator of NF-kB and stimulates differentiation and signaling pathways in clastic cell precursors. Binding of OPG to RANKL inhibits the genesis of clastic cells, thus preventing RANKL linkage to receptor activator of NF-kB. Recent studies have shown that mechanical strain plays an important role in the regulation of OPG synthesis and RANKL expression.
Peri-miniscrew implant crevicular fluid (MICF) is the inflammatory transudate that flows out via the miniscrew implant crevice. The biomarkers in the MICF are used to assess the host’s response to mechanical forces. Similar to a natural tooth, as the force is applied, inflammatory reactions begin in tissues around miniscrew implants. In contrast to natural teeth, inflammatory reactions should be strictly limited by preventing weakness in the stability.
In tooth movement as mechanical forces are applied, periodontal ligament cells produce cytokines during bone remodeling. Likewise, similar mechanisms take place in MICF when a force is exerted. The composition of MICF is similar to that of gingival crevicular fluid, and both are affected by bone modeling procedures. Whereas changes in gingival crevicular fluid lead to the destruction of periodontal tissues, changes in MICF cause destruction in peri-implant structures. Hence, it can be speculated that the elevations of cytokines and enzymes in MICF reflect biologic responses induced by mechanical stress.
To our knowledge, no published study has evaluated the RANKL and OPG levels around miniscrew implants as a response to orthodontic force application. Therefore, the purposes of this study were to determine MICF volume, RANKL and OPG levels, and the OPG/RANKL ratio, and to test the null hypothesis that there are no statistically significant differences in MICF RANKL, OPG levels, and OPG/RANKL ratio between loaded and unloaded miniscrew implants at different time intervals.
Material and methods
A power analysis established by G*Power software (version 3.0.10; Franz Faul, Universität Kiel, Kiel, Germany), based on a 1:1 ratio among the groups, with a sample size of 37 miniscrew implants, would give more than 85% power to detect significant differences with a 0.45 effect size and at the α = 0.05 significance level.
Twenty patients who required bilateral maxillary first premolar extractions and canine distalizations as a part of their orthodontic treatment were included in this study. All patients were in good general health with healthy periodontiums and generalized probing depths not exceeding 3 mm, with no radiographic evidence of periodontal bone loss. Patients who had antibiotic therapy within the past 6 months and used anti-inflammatory drugs in the month before the study did not participate. Written informed consents were obtained from all patients or the parents of those under 18 years of age.
The patients were randomly divided into 2 groups, loaded and unloaded miniscrew implants, according to the loading pattern of their implants. The simple randomization method was used, with the software producing the randomization lists (version 15.0; SPSS, Chicago, Ill). Two patients in the unloaded miniscrew group were excluded because of improper oral hygiene. The mean ages of the loaded (3 male, 7 female) and unloaded (3 male, 5 female) miniscrew implant groups were 18.15 ± 3.06 and 17.75 ± 0.68 years, respectively ( Table I ).
| Loaded | Unloaded | |
|---|---|---|
| Age (y) | 18.15 ± 3.06 | 17.75 ± 0.68 |
| Sex | 3 female, 7 male | 3 female, 5 male |
Initially, fixed preadjusted edgewise brackets with 0.018 × 0.025-in slots were placed in the maxillary arch. After leveling, a 0.016 × 0.022-in stainless steel archwire was placed, and the second maxillary premolars and the first molars were ligated together before canine distalization. A total of 36 titanium miniscrew implants (8 mm long, 1.6 mm diameter; Mitos, Istanbul, Turkey) were placed bilaterally into the interradicular bone between the maxillary second premolars and first molars in the attached gingiva below the mucogingival junction. To reduce root contact, the implants were placed in an oblique direction buccolingually, 30° to 40° to the long axis of the teeth in the maxillary posterior area, as described by Park. In the loaded miniscrew group, a 150-g distalization force delivered by 7-mm Sentalloy closed-coil springs (GAC International, Bohemia, NY) was applied horizontally between the miniscrew and the canine immediately after the insertion of the miniscrew implants. In the unloaded miniscrew group, the loading was performed 1 month later. MICF volume, total amounts and concentrations of OPG and RANKL, and the OPG/RANKL ratio were determined.
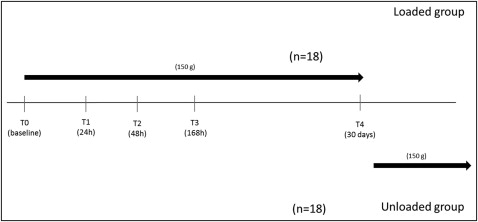
MICF sampling from both groups at each time point was performed by a periodontist (O.C.) according to the method of Uematsu et al with a slight modification. The samples were obtained before loading; at 24, 48, and 168 hours later; and on day 30 after force application. The timeline of the study is shown in Figure 1 .
After removal of plaque around the miniscrew implants, MICF samples were collected using paper strips (OraFlow, Plainview, NY) from the mesiobuccal aspects of the miniscrew implants. Each site was gently air-dried and isolated with cotton rolls, and paper strips were inserted into the crevice until mild resistance was felt. The paper strips were left for 30 seconds and transferred to an electronic gingival fluid measuring device (Periotron 8000; OraFlow) for volume determination. These samples were stored at −20°C and then transferred to −80°C until analysis. After 30 seconds of vortexing and 20 minutes of shaking, the strips were removed, and 200-μL eluted samples were assayed with enzyme-linked immunosorbent assay (ELISA) kits. MICF collection was standardized so that the loaded and unloaded sites and the subjects could be compared. Samples contaminated with saliva or blood were excluded. MICF samples were taken before all other clinical examinations were performed to prevent an increase in fluid volume.
The amounts of OPG and RANKL in the MICF samples were determined using commercially available human-specific ELISA in accordance with the manufacturers’ instructions (total RANKL ELISA kit; Raybiotech Inc, Norcross, Ga; OPG ELISA kit, USCN Life Science [USCNK] Inc, Houston, Tex). These assays measured the total levels of RANKL and OPG in the MICF, including both their unbound, free forms, and their OPG and RANKL complex forms. Calculation of the RANKL and OPG concentrations in each MICF sample was performed by dividing the total amount of RANKL or OPG by the volume of the sample: RANKL or OPG concentration (pg/μL) = total RANKL or OPG (pg)/volume (μL).
Statistical analysis
All statistical analyses were performed using software (SPSS). The Shapiro-Wilks normality test and the Levene variance homogeneity test were applied to the data, which did not show a normal distribution; there was no homogeneity of variances between groups. Intragroup comparisons were evaluated using the nonparametric Wilcoxon test. The statistical significance of intergroup differences was further assessed with the Mann-Whitney U test. The correlation among the MICF parameters was evaluated with the Spearman rank correlation test. The statistical significance level was set at P <0.05.
Results
All miniscrew implants survived until the end of study. There was a statistically significant increase in the total amount of OPG only at 168 hours ( P <0.05; Fig 2 ) in the unloaded miniscrew implant group, with no statistically significant differences in the loaded miniscrew implant group between all time periods compared with the baseline measurements. Similarly, there was no statistically significant difference in the total amount of RANKL in the groups at all time periods ( Fig 3 ) in comparison with the baseline. The results of the analysis indicated no statistically significant differences between the loaded and unloaded miniscrew implant groups for total amounts of OPG ( P >0.05), but the total amounts of RANKL were significantly elevated in the loaded miniscrew implant group ( P <0.05) with respect to the unloaded miniscrew implant group at all time periods. Both RANKL and OPG concentrations decreased at 48 hours in the loaded miniscrew implant group ( Table II ). The volume of MICF was elevated only at 48 hours in the loaded group ( Fig 4 ). Although the MICF OPG/RANKL ratio remained unchanged in the loaded miniscrew implant group, it increased in the unloaded group at the end of the observation period ( Fig 5 ).
| T0 | T1 | T2 | T3 | T4 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Median | Max | P | Min | Median | Max | P | Min | Median | Max | P | Min | Median | Max | P | Min | Median | Max | P | |
| Total amount of OPG (pg) | ||||||||||||||||||||
| Loaded | 11.34 | 16.58 | 41.43 | NS | 13.44 | 16.74 | 24.78 | NS | 12.76 | 17.01 | 31.69 | NS | 10.85 | 16.52 | 22.99 | NS | 13.13 | 17.04 | 22.62 | NS |
| Unloaded | 12.58 | 15.84 | 19.79 | 13.69 | 15.84 | 18.56 | 13.75 | 16.46 | 18.74 | 13.56 | 16.77 | 21.58 | 14.80 | 16.36 | 26.63 | |||||
| Total amount of RANKL (pg) | ||||||||||||||||||||
| Loaded | 455 | 988.75 | 1293 | ∗ | 437.5 | 982.5 | 1317 | ∗ | 382.5 | 953.75 | 1310 | ∗ | 490 | 986.25 | 1245 | ∗ | 587.5 | 956.25 | 1327 | ∗ |
| Unloaded | 417.5 | 607.5 | 1310 | 460 | 601.25 | 1157 | 447.5 | 691.25 | 1122 | 377.5 | 637.5 | 1227.5 | 432.5 | 637.50 | 987.5 | |||||
| Concentration of OPG (pg/μL) | ||||||||||||||||||||
| Loaded | 37.81 | 140.7 | 217.6 | NS | 30.98 | 72.74 | 191.7 | NS | 16.41 | 43.97 | 112.19 | ∗ | 13.56 | 73.66 | 172.6 | NS | 19.26 | 77.52 | 204.65 | NS |
| Unloaded | 27.64 | 130.69 | 171.98 | 52.19 | 81.06 | 168.9 | 28.97 | 74.13 | 173.23 | 28.15 | 75.67 | 167.68 | 53.63 | 87.38 | 188.03 | |||||
| Concentration of RANKL (pg/μL) | ||||||||||||||||||||
| Loaded | 2868.75 | 5168.75 | 12925 | NS | 1168 | 4631.25 | 12800 | NS | 1162 | 2471.25 | 6550 | NS | 1140 | 4212.5 | 10625 | NS | 1447 | 4456.25 | 13100 | NS |
| Unloaded | 933 | 4762.5 | 13100 | 1683 | 3935.42 | 7750 | 841 | 2568.75 | 7475 | 693 | 3187.5 | 9225 | 2162 | 2912.5 | 9875 | |||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


