Numerous simplified adhesives have been introduced to the dental market within the last few years, sometimes without comprehensive testing to validate the performance claimed by the respective manufacturers. Mild self-etch adhesives are unable to etch enamel to provide adequate retention for bonded restorations. Although high early resin–dentin bond strength values can be achieved with some self-etch adhesives, their resistance to thermal and mechanical stresses over time is disappointing. In light of the current drawbacks attributed to all-in-one self-etch adhesives, etch-and-rinse adhesives are still the benchmark for dental adhesion in routine clinical use. This article summarizes current issues and factors related to the performance of adhesives.
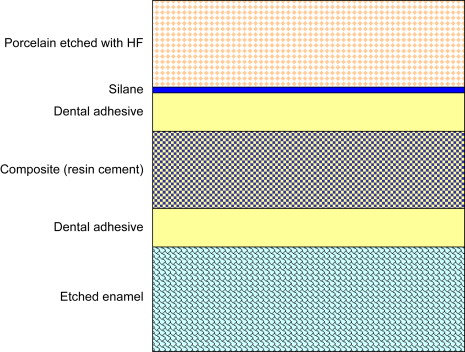
Adhesion or bonding is the process of forming an adhesive joint, which consists of two substrates joined together. In dentistry, the adherend is the substrate to which the adhesive—enamel and dentin, rarely cementum—is applied. Dental adhesives are solutions of resin monomers that join a restorative material with a dental substrate after the monomers set by polymerization. While most adhesive joints involve only two interfaces, dental adhesive joints may be more complex such as the enamel–adhesive–composite–adhesive–porcelain interface formed when a clinician bonds a porcelain restoration ( Fig. 1 ). A bonded composite restoration is another example of a complex adhesive joint.
In 1955, Buonocore reported the use of 85% phosphoric acid to improve the retention of an acrylic resin on enamel. The micromechanical nature of the interaction of dental adhesives with enamel is a result of the infiltration of resin monomers into the microporosities left by the acid dissolution of enamel and subsequent enveloping of the exposed hydroxyapatite crystals with the polymerized monomers within the pores on the enamel surface .
The ultimate goal of a bonded restoration is to attain an intimate adaptation of the restorative material with the dental substrate . This task is difficult to achieve as the bonding process for enamel is different from that for dentin. That is, dentin is more humid and more organic than enamel . While enamel is composed of 96% hydroxyapatite (mineral) by weight, dentin contains a significant amount of water and organic material, mainly type-I collagen . This humid and organic nature of dentin makes bonding to this hard tissue extremely difficult.
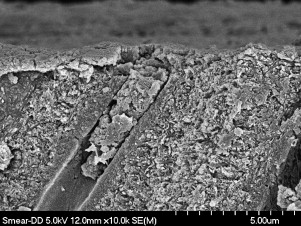
When tooth structure is cut with a bur or other instrument, the residual components form a “smear layer” of debris on the surface . This debris forms a uniform coating on enamel and dentin and plugs the entrance of the dentinal tubules, reducing the permeability of dentin ( Fig. 2 ). The smear layer is porous and permeable as a result of submicron channels that allow the dentinal fluid to pass through . The basic composition of the smear layer is hydroxyapatite and altered collagen with an external surface formed by gellike denatured collagen . The morphology of the smear layer is determined to a large extent by the type of instrument that creates it and by the site of dentin where it is formed .
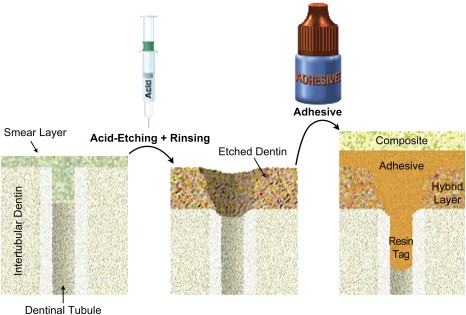
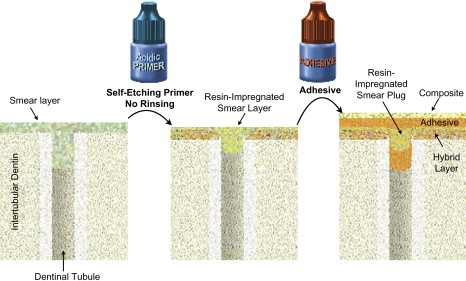
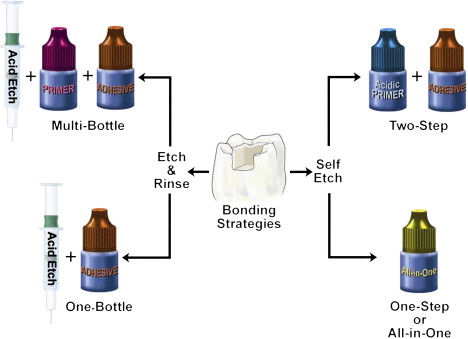
As the smear layer constitutes a true physical barrier, it must be dissolved or made permeable so the monomers in the adhesives can contact the dentin surface directly. In spite of different classifications of adhesive systems, the current adhesion strategies depend exclusively on how dental adhesives interact with this smear layer. One strategy involves etch-and-rinse adhesives, which remove the smear layer and superficial hydroxyapatite through etching with a separate acid gel ( Fig. 3 ). The second strategy involves self-etch adhesives, which make the smear layer permeable without removing it completely ( Figs. 4 and 5 ). Fig. 6 summarizes the current bonding strategies.
Etch-and-rinse strategy
With the etch-and-rinse strategy, dentin and enamel are treated with an acid gel (commonly phosphoric acid) to remove the smear layer and demineralize the most superficial hydroxyapatite crystals ( Fig. 7 ). Following this chemical etching, a mixture of resin monomers (primer/adhesive) dissolved in an organic solvent is applied to infiltrate etched dentin . The resin monomers permeate the water-filled spaces between adjacent dentin collagen fibers that used to be occupied by hydroxyapatite crystals. This infiltration results in a hybrid tissue composed of collagen, resin, residual hydroxyapatite, and traces of water (see Fig. 3 ) known as the resin–dentin interdiffusion zone, first described in 1982 as the hybrid layer . This intimate micromechanical entanglement of resin monomers with etched dentin may result in decreased postoperative sensitivity, may make for a better marginal fit, and may even act as an elastic buffer that compensates for the polymerization shrinkage stress during contraction of the restorative composite .

Self-etch strategy
The latest development in dental adhesion is based on simplification and reduced application time. These self-etch (nonrinsing) adhesives do not require a separate acid-etch step as they condition and prime enamel and dentin simultaneously by infiltrating and partially dissolving the smear layer and hydroxyapatite to generate a hybrid zone that incorporates minerals and the smear layer .
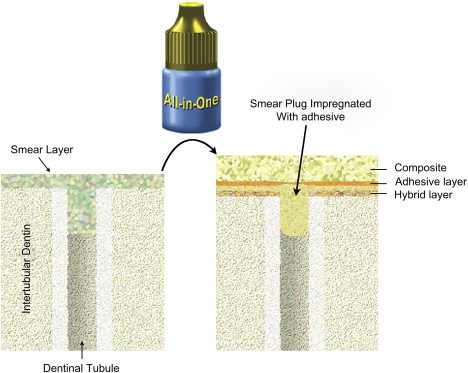
The first self-etch nonrinsing adhesives were composed of two solutions, an acidic primer and a bonding resin (see Fig. 4 ). Recently, many clinicians have shifted to one-step self-etch systems (also named all-in-one adhesives) in which manufacturers have attempted to incorporate all the primary components of an adhesive system (etchant, primer, and bonding resin) into a single solution (see Fig. 5 ). Both the acidic primer of two-step self-etch adhesives and the all-in-one solution are composed of aqueous mixtures of acidic functional monomers, generally phosphoric-acid or carboxylic-acid esters, with a pH higher than that of phosphoric acid gels . Water is an essential component of self-etch adhesives as it participates in the ionization of the acidic moieties.
All-in-one adhesives are user-friendly in that fewer steps are required for the bonding protocol. The elimination of separate etching and rinsing steps simplified the bonding technique, making these systems more popular in daily practice .
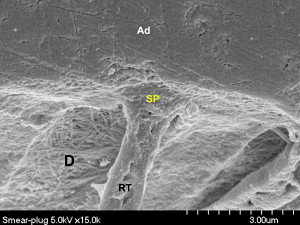
Self-etch adhesives differ in their aggressiveness. Therefore they are classified in three categories according to acidity: mild, moderate, and aggressive . Because self-etch adhesives are not as aggressive as the phosphoric-acid gel in etch-and-rinse adhesives, most do not remove the smear layer ( Fig. 8 ). Adper Prompt L-Pop (3M ESPE) is considered an aggressive self-etch adhesive (pH = 0.9–1.0), while other all-in-one adhesives, such as Clearfil S3 Bond, iBond, and G-Bond, are mild or moderate (pH >1.5) ( Table 1 ) .
| Adhesive (manufacturer) | pH |
|---|---|
| AdheSE (Ivoclar Vivadent) | 1.7 |
| Adper Prompt L-Pop (3M ESPE) | 0.9–1.0 |
| Clearfil S 3 Bond (Kuraray) | 2.4 |
| Clearfil SE Bond (Kuraray) | 1.8 |
| iBond (Heraeus Kulzer) | 2.2 |
| Xeno IV (Dentsply Caulk) | 2.5 |
Enamel bonding with self-etch adhesives
The enamel bond strengths of the earliest self-etch adhesives were lower than those associated with adhesives that rely on a separate etching step . Because of their higher pH, two-step self-etch adhesives result in shallower enamel demineralization compared with that of phosphoric acid . Nevertheless, roughening of enamel to remove prismless enamel improves the enamel-bonding ability of self-etch adhesives . A separate phosphoric-acid enamel-etching step also enhances the efficacy of self-etch adhesives . Two-step self-etch adhesives bond at an acceptable level to normal dentin and to ground enamel in vitro . Conversely, two-step self-etch materials may not bond as well to intact enamel and sclerotic dentin .
All-in-one self-etch systems are not as acidic as the phosphoric acid used with the etch-and-rinse adhesives . This characteristic has raised concerns about the performance of all-in-one self-etch systems on intact enamel ( Fig. 9 ). Several in vitro investigations have reported low resin–enamel bond strength of all-in-one self-etch materials . Grinding the enamel during a bevel or cavity preparation, for instance, makes the substrate more receptive for bonding with all-in-one self-etch systems .
Despite the increased popularity of self-etch adhesives, etching with phosphoric acid is still considered the golden standard against which new materials are tested .
Dentin bonding with self-etch adhesives
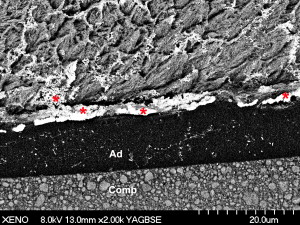
In spite of their user-friendliness and low technique sensitivity, all-in-one adhesives have resulted in low bonding effectiveness in vitro while their clinical reliability has often been questioned. Another drawback associated with all-in-one adhesives is their behavior as semipermeable membranes. These materials allow the movement of water across the bonded interface, which potentially leads to hydrolytic degradation ( Fig. 10 ) .
Consistent information regarding the durability of self-etch adhesives when applied on dentin is available in the literature . Although high early resin–dentin bond-strength values may be achieved with self-etch adhesives, their bonding effectiveness over time is disappointing . Because of the high hydrophilic nature of the acidic monomers and the high water concentration required for ionization of the acidic monomers in all-in-one self-etch solutions, these materials are likely to have resin–enamel bonds compromised over time. An inadequate resin penetration into tooth substrates may result in accelerated degradation of the structure of the bonding interface . As polymerization shrinkage stresses the bonding interface, dentin adhesives that do not resist these stresses result in low bond strengths, marginal gaps, recurrent caries, and pulpal irritation .
For some all-in-one adhesives, performance may depend on the application method, as the number of coats recommended by the manufacturer may not suffice . All-in-one adhesives have resulted in a wide range of dentin bond strengths . Application of the all-in-one adhesive in multiple layers may result in higher bond strengths and better infiltration into the hybrid layer . One manufacturer recommends rubbing the adhesive continuously for 15 seconds, followed by the application of a second coat after gentle air-drying and curing the first coat. This second coat prevents the formation of dry spots on the dentin surface and may result in better impregnation of the monomers into the hybrid layer, as observed in Figs. 11 and 12 .
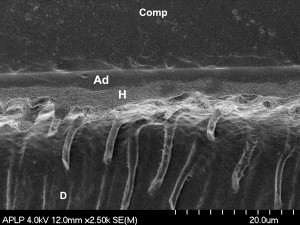

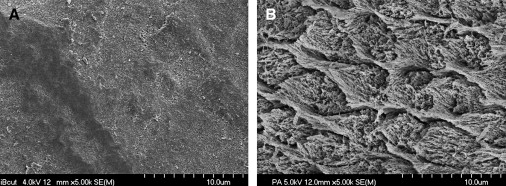
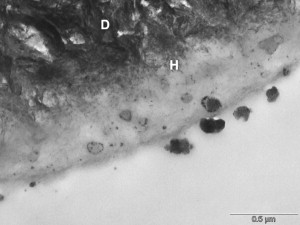
Low enamel and dentin bond strengths have been reported when acetone-based all-in-one adhesives are applied as per manufacturer’s directions . These adhesives result in severe enamel microleakage following thermal stresses . When applied to one-surface occlusal preparations, one of the most popular acetone-based all-in-one adhesives is not able to withstand polymerization shrinkage stresses nor thermocycling, resulting in a high percentage of pretesting debonds . Several mechanisms may account for this poor performance as compared with adhesives with different solvents. The magnitude of dentin bond strengths depends on the degree of infiltration of the resin monomers into the collagen pretreated with an acidic conditioner or with phosphoric acid . The author’s electron microscopy analyses ( Figs. 13 and 14 ) have demonstrated that acetone-based all-in-one adhesives result in a hybrid layer 0.2 to 0.5 μm thick, interfacial gaps, and limited resin penetration into the dentinal tubules. It is known that hybrid layers are particularly susceptible to degradation when the cavo-surface margins are not in enamel . The degradation of the dentin bonding interface is caused by the availability of exposed collagen fibrils at the base of hybrid layer or by hydrolytic degradation of resin components in the hybrid layer . Water can also infiltrate and plasticize the resin matrix, which decreases the mechanical properties of the polymer .
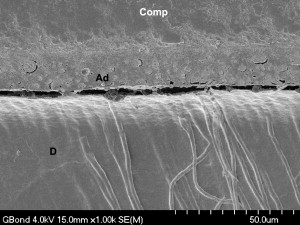

Other factors may play a role in the weak bonding performance of acetone-containing all-in-one adhesives. Porosities (or blisters) occur at the enamel and dentin bonding interfaces because most all-in-one adhesives behave as semipermeable membranes . These porosities may be a result of water accumulation either caused by an osmotic gradient or by monomer–solvent phase separation upon evaporation of the acetone . The number and size of these blisters may also depend on the intensity of the air-drying step .
High hydrophilicity, and consequent higher water sorption, has been reported associated with another acetone-based all-in-one adhesive containing a poly-phosphate molecule, dipentaerythritol penta-acrylate phosphate . The adhesive may undergo a substantial reduction in elastic modulus after water sorption, due to the plasticizing effect of water . If the adhesive has been plasticized by water, the restoration will likely fail when a load is applied on the surface because the stresses cannot be transferred across the adhesive joint. Another factor that may lead to lower bond strengths is the adhesive’s low degree of conversion, which has been demonstrated for specific adhesive compositions .
Clearfil SE Bond (Kuraray) ( Table 2 ), a two-step self-etch adhesive based on 10-methacryloyloxy decyl dihydrogen phosphate (10-MDP), is a reference for all other self-etch adhesives when it comes to dentin bond strengths . In the author’s laboratory, Clearfil S 3 Bond, the all-in-one successor of Clearfil SE Bond, shows lower dentin bond strengths than its predecessor. The pH of Clearfil S 3 Bond is less acidic than that of Clearfil SE Bond primer (2.4 versus 1.8, respectively). In spite of its relatively high pH, the bond strengths of Clearfil S 3 Bond on unground enamel are statistically similar to those of a more aggressive self-etch adhesive, Adper Prompt L-Pop (pH = 0.9–1.0) when tested in the author’s laboratory. The manufacturer of Clearfil S 3 Bond claims that the difference between the two 10-MDP–based adhesives may lie on the “molecular dispersion technology” that keeps the homogeneity of Clearfil S 3 Bond adhesive and prevents phase separation that occurs with acetone-based all-in-one adhesives . A recent study showed that Clearfil S 3 Bond is more resistant to mechanical stress than its two-bottle predecessor , which is surprising taking into consideration the excellent retention rates (above 90%) of Clearfil SE Bond in class-V clinical studies at 2 to 3 years . This clinical behavior of both Clearfil adhesives denotes a good resistance to clinical fatigue of adhesives containing 10-MDP.
| Adhesives and manufacturers | Composition | Instructions for use | Type | |
|---|---|---|---|---|
| AdheSE | Ivoclar Vivadent |
|
|
Two-step self-etch |
| Adper Prompt L-Pop | 3M ESPE | HEMA phosphates, HEMA, bis-GMA, modified polyalkenoic acid, water, photo-initiator |
|
All-in-one self-etch, but requires mixing |
| Adper Single Bond Plus | 3M ESPE | HEMA, bis-GMA, DMAs, methacrylate functional copolymer of polyacrylic and polyitaconic acids, water, ethanol, nanofiller, photo-initiator |
|
Two-step etch and rinse |
| Clearfil S 3 Bond | Kuraray America | 10-MDP, HEMA, bis-GMA, water, ethanol, silanated colloidal silica, camphorquinone |
|
All-in-one self-etch |
| Clearfil SE Bond | Kuraray America |
|
|
Two-step self-etch |
| G-Bond | GC America | 4-MET, UDMA, phosphate monomer, DMA component, fumed silica filler, acetone, water, photo-initiator |
|
All-in-one self-etch |
| iBond | Heraeus Kulzer | UDMA, 4-MET, glutaraldehyde, acetone, water, stabilizer, photo-initiator |
|
All-in-one self-etch |
| Xeno IV | Dentsply Caulk | UDMA, PENTA, acetone, polymerizeable trimethacrylate resin, and two polymerizeable DMA resins, photo-initiator. |
|
All-in-one self-etch |
| XP Bond | Dentsply DeTrey | Carboxylic acid modified dimethacrylate (TCB resin), PENTA, UDMA, TEGDMA, HEMA, butylated benzenediol (stabilizer), ethyl-4-dimethylaminobenzoate, camphorquinone, functionalized amorphous silica, t-butanol |
|
Two-step etch and rinse |
Clinical performance of all-in-one adhesives
Some manufacturers of all-in-one self-etch adhesives do not recommend enamel pre-etching, while other manufacturers recommend phosphoric-acid etching of unground enamel. One would argue that additional enamel bond strength is always required to prevent marginal gaps and leakage. In a clinical setting, the adhesive is extended past the boundaries of the preparation, forming composite flashes. Bonding to this uninstrumented surface is needed to resist potential staining. The manufacturer of Xeno IV (Dentsply) recommends beveling the enamel margins for all procedures, which may be difficult to achieve in the cervical margin of class-II preparations. The manufacturers of Adper Prompt L-Pop (3M ESPE) and iBond (Heraeus Kulzer) do not recommend a separate enamel-etching step. The manufacturer of Clearfil S 3 Bond (Kuraray) recommends phosphoric-acid etching of unground enamel. Pre-etching may improve bonding to uninstrumented surfaces for all mild all-in-one adhesives, especially in areas where an adhesive flash formed during insertion. When enamel is etched separately, bond strengths increase significantly for self-etch adhesives . However, if etching is extended to dentin, bonding may be compromised .
The enamel bond strengths of aggressive self-etch adhesives are usually higher than those of other self-etch adhesives , which may have to do with the relatively low pH compared with other less acidic adhesives (see Table 1 ). A recent morphological scanning electron microscopy study reported that the etching pattern on Adper Prompt, the bottle version of Adper Prompt L-Pop, is similar to that of phosphoric acid . Other all-in-one adhesives, such as G-Bond (GC America) and iBond (Heraeus Kulzer), do not result in any discernible etching pattern or minimal exposure of crystallites in sporadic areas of unground enamel. On ground enamel, these adhesives result in islands of superficially dissolved enamel within areas without any sign of enamel dissolution ( Fig. 15 ). One of the first clinical signs that a composite restoration is prone to failure is its early marginal staining. In fact, iBond is unable to penetrate the enamel smear layer , which explains why it is not very effective when applied on ground enamel. The almost inexistent etching pattern of iBond (an adhesive based on 4-methacryloxyethyl trimellitic acid (4-MET)) may have been responsible for massive marginal failure in a posterior composite clinical study at 1 year . The all-in-one adhesive iBond resulted in statistically worse marginal staining than any of the other adhesives used in this study. Two other adhesives, a 10-MDP–based all-in-one adhesive (Clearfil S 3 Bond) and a self-etch adhesive based on polyalkenoic acid copolymer and hydroxyethyl-methacrylate (Adper Prompt L-Pop) resulted in a slightly lower percentage of excellent margins than the etch-and-rinse adhesive One-Step Plus, but these differences were not significant. At 1 year, the restorations inserted with iBond showed a dark orange aspect in most of the restorations, which was already evident at 6 months ( Figs. 16 and 17 ). The change in color of the restoration might have been a result of the degradation of the adhesive itself, not that of the composite material, as the other adhesives did not result in such catastrophic results. This color change of the 4-MET–based all-in-one adhesive may be a result of its rapid hydrolysis, which is temperature-dependent and occurs as early as at 1 month, when specimens are stored at 25°C to 37°C .