Dental plaque is the biofilm found naturally on teeth. Dental plaque is also implicated in dental caries, which is associated with shifts in the microbial balance of the biofilm resulting in increased proportions of acid producing and acid tolerating bacteria, especially (but not exclusively) mutans streptococci and lactobacilli. The regular intake of fermentable dietary sugars, or impaired saliva flow, produces persistent conditions of low pH within the biofilm, which selects for these cariogenic bacteria. Clinicians should prevent this disruption to the natural microbial balance of the biofilm (relevant approaches are described) rather than merely treating its consequences by restoring cavities.
Humans have an intimate and dynamic relationship with microorganisms. The human body is estimated to be composed of more than 10 14 cells, of which only 10% are mammalian. The majority are the microorganisms that make up the resident microfloras that colonize all exposed surfaces of the body. The microfloras of the skin, mouth, and digestive and reproductive tracts are distinct from each other despite the frequent transfer of organisms between these sites; their characteristic composition is due to significant differences in the biologic and physical properties of each habitat. These properties determine which microorganisms are able to colonize and which predominate or have only a minor role. This observation illustrates a key concept, namely, that the properties of the habitat are selective and dictate which organisms are able to colonize, grow, and be minor or major members of a microbial community.
The resident microfloras of the host not only reside passively at a site but also make an active contribution to the maintenance of health by (1) promoting the normal development of the immune system—some members of the resident microflora might also play a role in damping down deleterious immune responses, and (2) excluding exogenous (and often pathogenic) microorganisms. This latter process (colonization resistance) is due to the resident oral microflora being more competitive in terms of nutrient acquisition and attachment to oral receptors and by producing inhibitory molecules.
The resident oral microflora
The mouth is similar to other habitats in the body in possessing a diverse but characteristic resident microbial community. Bacteria are the most numerous group and, initially, they were characterized solely using cultural techniques. The recent application of molecular approaches that do not depend on prior cultivation for identification has provided deeper insights into the true richness of the resident oral microflora. It is now estimated that there are more than 700 different types of microbe that can be isolated from the mouth but that greater than 50% of these cannot currently be grown in pure culture in the laboratory.
The resident oral microorganisms obtain their nutrients primarily from endogenous sources, such as amino acids, proteins and glycoproteins in saliva, and gingival crevicular fluid; the metabolism of these substrates leads to only minor and slow changes to the local pH. Saliva also plays a major role in maintaining the oral pH at approximate neutrality, which is optimal for the growth of the majority of the microorganisms associated with oral health. In contrast (discussed later), diet has a limited but generally deleterious impact on the balance of the resident oral microflora, mediated mainly by the rapid falls in pH in dental plaque.
The composition of the oral microflora varies significantly at distinct surfaces within the mouth (eg, tongue, buccal mucosa, and teeth), again due to differences in key environmental conditions. This is despite the opportunity that bacteria have to colonize each site, and this observation further emphasizes the link that exists between the properties of the habitat and the organisms that are able to become established and predominate.
The resident microfloras on mucosal and dental surfaces in the mouth are examples of microbial biofilms. Biofilms are 3-D accumulations of interacting microorganisms attached to a surface, embedded in a matrix of extracellular polymers. Research over the past decade has demonstrated that the properties of microbes attached to a surface as a biofilm are different from those expressed when the same organisms are grown under conventional conditions in a laboratory in liquid media. Of clinical relevance is the property that biofilms display increased tolerance to antimicrobial agents and to the host defenses.
Dental plaque biofilms
The most diverse collections of oral microorganisms are found in the biofilms on teeth (dental plaque). A small sample of dental plaque contains, on average, between 12 and 27 species. These biofilms develop in a specific pattern. Within seconds of eruption, or after cleaning, tooth surfaces become coated with a conditioning film of molecules (biologically active proteins and glycoproteins) derived mainly from saliva (and also from gingival crevicular fluid and from the bacteria themselves). Initially, only a few bacterial species are able to attach to this film, which is also termed, the acquired pellicle . Cells are held reversibly near to the surface by weak, long-range physicochemical forces. Molecules (adhesins) on these early bacterial colonizers, mainly streptococci (eg, Streptococcus mitis and S oralis ) can bind to complementary receptors in the acquired pellicle to make the attachment irreversible and then these pioneer species start to multiply. The metabolism of the early colonizers modifies the local environment, for example, by making it more anaerobic after their consumption of oxygen. As the biofilm develops, adhesins on the cell surface of more fastidious secondary colonizers, such as obligate anaerobes, bind to receptors on already attached bacteria by a process termed, coadhesion or coaggregation , and the composition of the biofilm becomes more diverse ( microbial succession ). The attached bacteria produce extracellular polymers (the plaque matrix) that consolidate attachment of the biofilm. The matrix is more than a mere scaffold for the biofilm; the matrix can bind and retain molecules, including enzymes, and also retard the penetration of charged molecules into the biofilm. Biofilms are spatially and functionally organized, and the heterogeneous conditions within the biofilm induce novel patterns of bacterial gene expression, while the close proximity of different species provides the opportunity for interactions. Examples of these interactions include (1) the development of food chains (in which the end product of metabolism of one organism is used by secondary feeders) and metabolic cooperation among species to catabolize structurally complex host macromolecules; these interactions increase the metabolic efficiency of the microbial community; (2) cell-cell signaling, for example, by the secretion of small peptides to coordinate gene expression among cells of a similar species; (3) transfer of antibiotic resistance genes, and (4) antagonism by the production of inhibitory molecules, which may provide a competitive advantage to the producing organism or exclude undesirable microbes.
Thus, dental plaque is a classic example of a biofilm and a microbial community, in which bacteria interact and the properties of the whole consortium are more than the sum of the constituent species. Additional information can be found in review articles that describe more fully the significance of dental plaque as a multispecies biofilm.
The microbial composition of the biofilm varies at distinct sites on a tooth (fissures, approximal surfaces, and gingival crevice) and reflects the inherent differences in their anatomy and biology. The normal microflora of fissures is sparse and the organisms present have a saccharolytic metabolism (ie, their energy is derived from sugar catabolism); the predominant bacteria are streptococci and there are few gram-negative or anaerobic organisms. In contrast, the gingival crevice has a more diverse microflora, including many gram-negative anaerobic and proteolytic species, whereas approximal surfaces have a microflora that is intermediate in composition.
Once established, the composition of the resident microflora at any site remains stable over time, unless there are marked changes to the habitat. This stability, termed microbial homeostasis , stems not from any metabolic indifference by the resident microflora but reflects a highly dynamic state in which the proportions of individual species are in balance due to the many interactions, both synergistic and antagonistic (described previously). For example, long-term use of broad-spectrum antibiotics can suppress the resident bacterial oral microflora permitting overgrowth by previously minor populations of oral yeasts. This clinical observation demonstrates 2 principles. First, the resident oral microflora is responsive to environmental change and a major shift in local conditions can drive alterations in the composition and metabolic activity of the microflora that are deleterious to the health of the host, and, second, oral care practices should attempt to maintain plaque at levels compatible with health to retain the beneficial properties of the normal oral microflora.
Dental plaque biofilms
The most diverse collections of oral microorganisms are found in the biofilms on teeth (dental plaque). A small sample of dental plaque contains, on average, between 12 and 27 species. These biofilms develop in a specific pattern. Within seconds of eruption, or after cleaning, tooth surfaces become coated with a conditioning film of molecules (biologically active proteins and glycoproteins) derived mainly from saliva (and also from gingival crevicular fluid and from the bacteria themselves). Initially, only a few bacterial species are able to attach to this film, which is also termed, the acquired pellicle . Cells are held reversibly near to the surface by weak, long-range physicochemical forces. Molecules (adhesins) on these early bacterial colonizers, mainly streptococci (eg, Streptococcus mitis and S oralis ) can bind to complementary receptors in the acquired pellicle to make the attachment irreversible and then these pioneer species start to multiply. The metabolism of the early colonizers modifies the local environment, for example, by making it more anaerobic after their consumption of oxygen. As the biofilm develops, adhesins on the cell surface of more fastidious secondary colonizers, such as obligate anaerobes, bind to receptors on already attached bacteria by a process termed, coadhesion or coaggregation , and the composition of the biofilm becomes more diverse ( microbial succession ). The attached bacteria produce extracellular polymers (the plaque matrix) that consolidate attachment of the biofilm. The matrix is more than a mere scaffold for the biofilm; the matrix can bind and retain molecules, including enzymes, and also retard the penetration of charged molecules into the biofilm. Biofilms are spatially and functionally organized, and the heterogeneous conditions within the biofilm induce novel patterns of bacterial gene expression, while the close proximity of different species provides the opportunity for interactions. Examples of these interactions include (1) the development of food chains (in which the end product of metabolism of one organism is used by secondary feeders) and metabolic cooperation among species to catabolize structurally complex host macromolecules; these interactions increase the metabolic efficiency of the microbial community; (2) cell-cell signaling, for example, by the secretion of small peptides to coordinate gene expression among cells of a similar species; (3) transfer of antibiotic resistance genes, and (4) antagonism by the production of inhibitory molecules, which may provide a competitive advantage to the producing organism or exclude undesirable microbes.
Thus, dental plaque is a classic example of a biofilm and a microbial community, in which bacteria interact and the properties of the whole consortium are more than the sum of the constituent species. Additional information can be found in review articles that describe more fully the significance of dental plaque as a multispecies biofilm.
The microbial composition of the biofilm varies at distinct sites on a tooth (fissures, approximal surfaces, and gingival crevice) and reflects the inherent differences in their anatomy and biology. The normal microflora of fissures is sparse and the organisms present have a saccharolytic metabolism (ie, their energy is derived from sugar catabolism); the predominant bacteria are streptococci and there are few gram-negative or anaerobic organisms. In contrast, the gingival crevice has a more diverse microflora, including many gram-negative anaerobic and proteolytic species, whereas approximal surfaces have a microflora that is intermediate in composition.
Once established, the composition of the resident microflora at any site remains stable over time, unless there are marked changes to the habitat. This stability, termed microbial homeostasis , stems not from any metabolic indifference by the resident microflora but reflects a highly dynamic state in which the proportions of individual species are in balance due to the many interactions, both synergistic and antagonistic (described previously). For example, long-term use of broad-spectrum antibiotics can suppress the resident bacterial oral microflora permitting overgrowth by previously minor populations of oral yeasts. This clinical observation demonstrates 2 principles. First, the resident oral microflora is responsive to environmental change and a major shift in local conditions can drive alterations in the composition and metabolic activity of the microflora that are deleterious to the health of the host, and, second, oral care practices should attempt to maintain plaque at levels compatible with health to retain the beneficial properties of the normal oral microflora.
Dental plaque and caries disease
Many studies have been undertaken to determine the composition of biofilms from sites with caries lesions to try and identify the bacteria responsible for causing the demineralization. Interpretation of data from such studies is difficult because plaque-mediated diseases occur at sites with a pre-existing natural and diverse resident microflora. The anatomy of sites at risk for caries lesions means that there are intrinsic difficulties in taking discrete plaque samples. Traditional culture techniques have generally been applied to determine the bacterial composition of the plaque samples, but these approaches do not recover all of the microorganisms that are present, so potentially significant species could be underestimated or missed. Bifidobacteria are now being linked to caries disease etiology, but these bacteria have been difficult to isolate until recently when an effective selective medium for their detection was introduced. There are wide intersubject variations in the composition of the plaque microflora from the same site, so that when data are averaged from many individuals, clear associations between bacteria and disease can be difficult to discern. In addition, the traits associated with cariogenicity (acid production, acid tolerance, and intracellular and extracellular polysaccharide production) are not restricted to a single species (discussed later). Similarly, the consequence of acid production by cariogenic species can be ameliorated by the development of food chains with other plaque bacteria, such as Veillonella spp (which convert lactate to weaker acids), or due to alkali production (eg, ammonia generation from arginine or urea metabolism) by neighboring organisms.
Historical Perspective
Despite all these issues, progress has been made in determining the bacterial etiology of dental caries disease. In the late nineteenth century, Dr W.D. Miller put forward the “chemico-parasitic” theory of caries disease, in which he proposed that oral microorganisms can break down dietary carbohydrates to acids which demineralize enamel. Microbiology was in its infancy, however, and it was not possible to determine which bacteria were involved. In 1924, Clarke isolated streptococci from human caries lesions and named them S mutans . This finding was overlooked, however, for several decades, and it was not until the 1960s that further substantial progress was made when gnotobiotic animal studies were feasible and it could be shown categorically that (1) caries disease was a transmissible, (2) fermentable carbohydrates in the diet played a critical role, (3) oral streptococci (and other bacteria) from humans could cause caries lesions in rodents fed a high sugar diet, and (4) interventions, such as antibiotics targeted against these bacteria, prevented caries lesions. The most cariogenic species in these animal studies were what are now termed mutans streptococci, in particular S mutans and S sobrinus . These studies laid the foundation for epidemiologic studies in humans in which the prevalence and proportions of selected bacteria or the whole plaque microflora were compared at caries versus healthy surfaces. Detailed studies of the biochemistry and molecular biology of cariogenic bacteria have enabled the traits associated with cariogenicity to be identified. These include (1) the expression of high-affinity sugar transport systems for the uptake of fermentable carbohydrates and the rapid conversion of the transported sugars to acidic end products of metabolism (acidogenicity); (2) the ability to tolerate, grow, and continue to make acid in low-pH environments (aciduricity); (3) the synthesis of extracellular polymers (especially glucans and mutan) from sucrose to consolidate attachment; and (4) the production of intracellular polysaccharides during periods of excess carbohydrate availability; these storage compounds can be converted to acid during periods between meals when dietary sugars are not available.
Human Epidemiologic Study Design
Two types of epidemiologic survey have been performed to determine the microbial etiology of caries disease. In cross-sectional surveys, predetermined caries-prone surfaces are sampled at a single time point, and the plaque microflora is related to the caries status of the site at that time. Large numbers of sites/people can be analyzed, and different age groups, tooth surfaces, diets, intervention strategies, and so forth can be compared. A major disadvantage of this study design, however, is that it cannot be determined for certain whether or not the species that are isolated from caries surfaces caused the decay or arose because of it; only associations can be derived from this study design. In contrast, longitudinal studies sample initially clinically sound sites at regular intervals over a set time period. Sites are selected on the basis of previous epidemiologic surveys, from which it can be predicted that a statistically relevant number of sites should suffer caries lesions within the time span of the study. The microflora can then be compared (1) before and after the diagnosis of disease and (2) between those sites that became diseased and those that remained healthy throughout the study, so that true cause-and-effect relationships can be established. These studies take longer to perform and are far more resource demanding (ie, expensive), so, for practical reasons, far fewer longitudinal studies have been performed.
Main Microbiologic Findings From Human Epidemiologic Studies
It is beyond the scope of this article to review the results from all of the studies performed on humans; more comprehensive reviews are recommended to readers. Instead, data from some typical studies are highlighted to indicate the main trends.
Fissures are the most prone sites for caries lesions of the dentition, and the strongest correlation between the plaque levels of mutans streptococci and caries lesions has been found on these surfaces. For example, in a typical cross-sectional study, 71% of single fissures with open caries lesions in US children with rampant caries (and living in an area with a nonfluoridated water supply) had viable counts of mutans streptococci greater than 10% of the total cultivable plaque microflora, whereas 70% of fissures from similarly aged children who did not have visible caries lesions (but living in a community with water fluoridation) had no detectable mutans streptococci. It was possible, however, to have a caries lesion in a fissure without detectable mutans streptococci as well as to find high levels of these bacteria in fissures that were diagnosed as being sound. In the same study, 65% of pooled plaque samples (approximal and occlusal surfaces of the 2 most posterior teeth) from children with rampant caries had counts of mutans streptococci greater than 10% of the plaque microflora whereas 40% of similar samples from children without any visible caries lesions had no detectable mutans streptococci. However, 16% of the pooled plaque samples from children with rampant caries had only low levels of mutans streptococci (<1% of the total plaque microflora).
In a longitudinal study of fissures in 52 US patients (aged 5 to 12 years) in which 4 examinations were performed at 6-month intervals, the proportions of mutans streptococci increased significantly at the time of lesion diagnosis. The proportions of mutans streptococci reached almost 25% of the total fissure plaque in high caries active children at the time of diagnosis (compared with 7% and 10% at 12 and 6 months, respectively, before caries disease diagnosis). This trend was not observed in sound sites or in fissures that developed caries lesions but were in a low caries active group. Mutans streptococci were only minor components of plaque from 5 fissures that became carious, but these sites had high levels of lactobacilli, and these bacteria may have been responsible for the observed demineralization. A subsequent longitudinal study confirmed these findings and demonstrated an even stronger relationship between mutans streptococci and caries lesion initiation whereas lactobacilli, when present, were strongly associated with sites requiring restoration.
A major prospective study of young Swiss children (aged 7–8 years) found that fissures and smooth surfaces of first permanent premolars that suffered demineralization without cavitation were heavily colonized with mutans streptococci (10 4 –10 5 colony forming units/mL sample) at approximately 12 to 18 months before the clinical diagnosis of the lesion. The proportions of mutans streptococci markedly increased 6 to 9 months before lesion detection, reaching 11% to 18% and 10% to 12% of the total streptococcal microflora of fissures and smooth surfaces, respectively. As with most studies on the microflora of caries disease, this study found some fissures with high levels of mutans streptococci but no discernible lesion, whereas other sites that had caries lesions had no detectable mutans streptococci at any time.
Challenges for studies of approximal surfaces lie with the difficulty in accurately detecting early lesions and with the fact that the biofilm is inevitably removed from the whole interproximal area, including that overlying sound as well as carious enamel. Early cross-sectional studies reported a positive correlation between elevated mutans streptococci levels and lesion development. A less clear-cut association was found in a large longitudinal study of 11- to 15-year-old UK children. At some sites, mutans streptococci could be found in high numbers before the radiographic detection of demineralization, whereas some lesions also developed in the apparent absence of these bacteria. Mutans streptococci could also be present at some sites for prolonged periods in high numbers without any evidence of caries lesion development. The isolation frequency and proportions of mutans streptococci tended to increase after the first diagnosis of a lesion, especially in those that progressed deeper into the enamel, suggesting that the composition of the microflora might shift as the lesion progresses through the structure of the tooth. In a study in The Netherlands, mutans streptococci were isolated from 40% of sound sites and 86% of sites with caries lesions in Dutch army recruits aged 18 to 20 years, respectively. In this study, S sobrinus (originally reported as S mutans serotype d ) was recovered almost exclusively from recruits with caries lesions.
Rampant caries can occur in people who experience an exceptional change in the oral environment, such as those with markedly reduced salivary flow rates due to, for example, radiation therapy or medication. Longitudinal studies of patients undergoing radiation treatment showed large increases in the proportions of mutans streptococci and lactobacilli in plaque and saliva. These organisms also reach high levels in nursing bottle caries (early childhood caries), which occurs in young infants fed from bottles containing formula with a high concentration of fermentable carbohydrate.
Collectively, the data from many surveys of various tooth surfaces, different patient age groups from many countries, populations with different dietary habits, and so forth, using conventional culture techniques, have shown a strong positive association between increased levels of mutans streptococci and the initiation of demineralization. This relationship is not absolute, however, and the majority of these epidemiologic studies report sites with mutans streptococci that are sound as well as surfaces that develop caries lesions in the apparent absence of mutans streptococci. In the latter samples, lactobacilli, bifidobacteria, Actinomyces spp, and low-pH non-mutans streptococci (streptococci that can generate acid and thrive at a low pH) have been implicated in caries lesion development.
Contemporary studies have used molecular approaches to detect the predominant bacteria present in biofilms overlying lesions, without the bias introduced by the culturing of organisms on selective and nonselective agar plates. These studies generally have recovered a more diverse microflora, and novel taxa have been described. One study confirmed the previously reported relationship of S sanguinis with sound enamel and S mutans and lactobacilli with caries lesions but additionally found Actinomyces gerencseriae and other Actinomyces spp implicated in caries lesion initiation and Bifidobacterium spp with advanced lesions. Another study reported that 10% of subjects with rampant caries in the secondary dentition did not have detectable levels of S mutans . In lesions where mutans streptococci could not be detected, there were high levels of lactobacilli, low pH–tolerating non-mutans streptococci, and Bifidobacterium spp. High levels of Actinomyces species and nonmutans streptococci were found in early (white spot) lesions, whereas mutans streptococci and lactobacilli, together with Propionibacterium and Bifidobacterium spp, dominated advanced lesions. Often these detailed molecular studies have been performed on small numbers of samples, and more extensive investigations are awaited. A consistent trend is again emerging from these culture-independent studies in which, although mutans streptococci and lactobacilli are often associated with lesions, they are not always present, and a more diverse microflora is implicated.
Hypotheses to Explain the Role of Plaque Bacteria in the Etiology of Dental Caries Disease
From the start of the discipline of microbiology, it has been recognized that dental plaque biofilms have a diverse microflora. Therefore, it was a major advance when the specific plaque hypothesis was put forward. This hypothesis proposed that only a few of the many species found in dental plaque biofilms are actively involved in disease. Thus, caries disease could be controlled by targeting preventative measures and interventions against these “specific” organisms, and the evidence at the time strongly implicated mutans streptococci as the main etiologic agent. Over time, as more studies identified sites where caries lesions developed in the absence of mutans streptococci, and there became a greater understanding of the metabolism of other members of the biofilm (eg, the role of bacteria that consumed acid or produced alkali), an alternative view on the role of plaque in caries lesion development was articulated. The nonspecific plaque hypothesis proposed that disease is the outcome of the overall activity of the total plaque microflora (as opposed to specific species), and, although often used in the context of periodontal diseases, the concept was also applied to caries disease. The arguments about the merits of both hypotheses may seem to be about semantics, because plaque-mediated diseases are essentially polymicrobial infections in which only a few (perhaps specific) species are able to predominate. These hypotheses, however, also have implications for treatment strategies. More general preventative approaches are warranted if a range of bacteria are involved whereas more specific interventions (targeted antimicrobials, vaccination) are justified if there is a more specific cause.
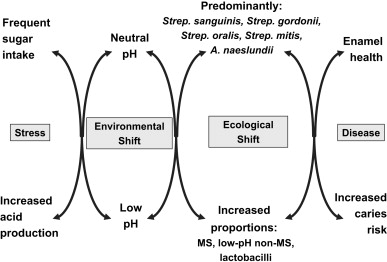
More recently, an alternative hypothesis was proposed (the ecological plaque hypothesis), which attempted to reconcile the key elements of the earlier 2 hypotheses and highlighted the critical role played by changes to the oral environment in predisposing an individual to caries disease. Dental caries disease is viewed as a consequence of an imbalance in the resident microflora due to the enrichment within the microbial community of potentially more highly cariogenic bacteria due to frequent conditions of low pH in plaque biofilms, for example, as a result of a change to the diet or a reduction in saliva flow ( Fig. 1 ). The application of more sensitive diagnostic methods has resulted in the frequent detection of mutans streptococci in plaque from healthy sites, albeit often in low numbers. These organisms are only weakly competitive with other oral bacteria at neutral pH and are present, therefore, as a small proportion of the total plaque community. In this situation, with a conventional diet, the levels of such potentially cariogenic bacteria are clinically insignificant, and the processes of de- and remineralization are in equilibrium. If the frequency of fermentable carbohydrate intake increases, then plaque spends more time below the critical pH for enamel demineralization (approximately pH 5.5). The effect of this on the microbial ecology of plaque is 2-fold. Conditions of low pH favor the proliferation of acid-tolerating (and acidogenic) bacteria (including mutans streptococci, lactobacilli, and other bacteria with a similar phenotype) while tipping the balance toward demineralization (see Fig. 1 ). Greater numbers of bacteria, such as mutans streptococci and lactobacilli, in plaque result in more acid being produced at even faster rates, thereby enhancing demineralization and further disrupting the ecology of the biofilm. Other bacteria could also make acid under similar conditions, but at a slower rate, but could be responsible for the initial stages of demineralization or could cause lesions in the absence of more overt cariogenic species in a more susceptible host. If aciduric species were not present initially, then the repeated conditions of low pH coupled with the inhibition of competing organisms might increase the likelihood of successful colonization by mutans streptococci or lactobacilli. This sequence of events would account for the lack of total specificity in the microbial etiology of caries disease and explain the pattern of bacterial succession often reported during lesion progression.