Reconstruction of the mandible is by definition restoring form and function to the mandibular bone and its dentition as well as its investing soft tissue envelope. This may be accomplished by a wide variety of procedures depending on, among other things, the etiology and extent of the deformity. Restoring form and function for a congenital deformity is obviously approached in a much different manner than a composite segmental defect acquired as a result of a cancer operation. Recognizing that there are too numerous techniques suitable for each potential clinical situation to discuss in this chapter, it will therefore focus on the most common, namely, reconstruction of acquired segmental defects of the mandible with nonvascularized grafts or vascularized bone flaps.
Etiopathogenesis/Causative Factors
Acquired segmental defects of the mandible are most commonly secondary to ablative tumor therapy or avulsive traumatic injury. Other less common causes include inflammatory or infectious conditions that result in devitalization of the mandibular bone requiring its debridement. Segmental defects secondary to tumor therapy may result from the management of aggressive benign tumors arising within the mandible such as ameloblastoma or myxoma or from malignancies (carcinomas/sarcomas) arising in the associated soft tissue envelope that invade or extend to the mandible. Management of oral squamous cell carcinoma is the most common malignancy resulting in acquired segmental defects of the mandible.
Avulsive segmental wounds most commonly arise from high-velocity injuries such as firearms, industrial accidents, and occasionally motor vehicle collisions. Fortunately, most traumatic injuries to the jaws do not result in segmental defects because of the lower kinetic energies associated with the injury. Kinetic energy (KE = mv 2 ) increases dramatically as the speed of the missile or the speed of impact increases, resulting in comminution of bone, destruction of the soft tissue envelope, and devitalization of large areas of bone and soft tissue. The extent of devitalization from high-velocity injuries may not be completely apparent at the time of presentation. The astute clinician will recognize the potential for extensive tissue loss in these patients and utilize wound care and temporary stabilization of the wound until all tissue loss has had opportunity to declare itself prior to definitive reconstruction.
Inflammatory conditions that result in devitalization of mandibular bone can be quite destructive. Osteoradionecrosis and bisphosphonate osteonecrosis represent two of the most common etiologies and may present with or without an associated acute osteomyelitis of the involved bone. Osteoradionecrosis results from a profound hypovascularity of the bone secondary to radiotherapy, whereas bisphosphonate osteonecrosis results from dysfunction of the osteoclasts caused most commonly by intravenous bisphosphonate therapy. The management of these specific etiologies is beyond the scope of this chapter; however, in the author’s experience, large segmental defects resulting from either process are amenable to reconstruction with vascularized fibular bone grafts.
Pathologic Anatomy/Classification of Defects
The location of the mandibular defect has a direct implication to management. Complex classification schemes have been proposed to help guide therapy based on bony, soft tissue, and neurologic deficits. The author prefers a simplified approach based on the position of the defect relative to the mandibular anatomy. This scheme divides the mandible into functional segments consisting of the symphysis (anterior) unit, the body (lateral) unit, the ramus-condyle (posterior) unit, and the alveolus ( Box 60-1 ).
-
Alveolar defects . Loss of alveolar segment without loss of mandibular continuity.
-
Anterior defects . Segmental defect incorporating the region of the mandibular symphysis or extending from cuspid to cuspid.
-
Lateral defects . Segmental defect involving the mandibular body region or extending from the mandibular cuspid to the retromolar region.
-
Posterior defects . Segmental defects involving the ramus and mandibular angle with or without loss of the condylar process.
Each of these sites has variable physiologic factors that result in different functional deficits when lost as a result of tumor ablation or traumatic disruption. Each region is also subject to significantly different forces (torsion, tension, compression) acting across the defect during function. Furthermore, numerous studies have evaluated failure rates for various techniques for mandibular reconstruction and identified differences based on the location of the defect. Consequently, reconstruction of each anatomic unit demands an individualized approach based on factors related to the patient, the reconstructive procedure, and the disease process. The impact of defect location and consideration of reconstructive technique will be further discussed later in the text.
A second factor for consideration is the importance of the soft tissue envelope. Evaluation of the soft tissue envelope should consider both the quality of the tissues and the quantity of tissue remaining or removed. Kazanjian in 1952 identified four essential factors for successful bone grafting, the first two of which were (1) implantation into healthy tissue and (2) good blood supply in the recipient area. Wounds that lack healthy soft tissue because of extensive trauma to the tissues or large volume loss of soft tissue as a result of resection are poor candidates for immediate nonvascular bone grafting. Likewise, wounds that are hypovascular due to radiotherapy or extensive scarring will provide poor wound beds for successful nonvascular bone grafting. In these situations, transfer of healthy vascularized tissue is essential for successful reconstruction. Complication rates for mandibular reconstruction have shown a tendency to increase with the increasing soft tissue resection. Loss of a significant volume of tissue precludes immediate nonvascular bone grafting in most situations. Failure rates with immediate nonvascular bone grafting techniques have been reported as high as 50% in such situations. Therefore, when a segmental defect is associated with extensive loss of soft tissues, immediate reconstruction with vascularized tissue transfer or delayed reconstruction should be considered.
Pathologic Anatomy/Classification of Defects
The location of the mandibular defect has a direct implication to management. Complex classification schemes have been proposed to help guide therapy based on bony, soft tissue, and neurologic deficits. The author prefers a simplified approach based on the position of the defect relative to the mandibular anatomy. This scheme divides the mandible into functional segments consisting of the symphysis (anterior) unit, the body (lateral) unit, the ramus-condyle (posterior) unit, and the alveolus ( Box 60-1 ).
-
Alveolar defects . Loss of alveolar segment without loss of mandibular continuity.
-
Anterior defects . Segmental defect incorporating the region of the mandibular symphysis or extending from cuspid to cuspid.
-
Lateral defects . Segmental defect involving the mandibular body region or extending from the mandibular cuspid to the retromolar region.
-
Posterior defects . Segmental defects involving the ramus and mandibular angle with or without loss of the condylar process.
Each of these sites has variable physiologic factors that result in different functional deficits when lost as a result of tumor ablation or traumatic disruption. Each region is also subject to significantly different forces (torsion, tension, compression) acting across the defect during function. Furthermore, numerous studies have evaluated failure rates for various techniques for mandibular reconstruction and identified differences based on the location of the defect. Consequently, reconstruction of each anatomic unit demands an individualized approach based on factors related to the patient, the reconstructive procedure, and the disease process. The impact of defect location and consideration of reconstructive technique will be further discussed later in the text.
A second factor for consideration is the importance of the soft tissue envelope. Evaluation of the soft tissue envelope should consider both the quality of the tissues and the quantity of tissue remaining or removed. Kazanjian in 1952 identified four essential factors for successful bone grafting, the first two of which were (1) implantation into healthy tissue and (2) good blood supply in the recipient area. Wounds that lack healthy soft tissue because of extensive trauma to the tissues or large volume loss of soft tissue as a result of resection are poor candidates for immediate nonvascular bone grafting. Likewise, wounds that are hypovascular due to radiotherapy or extensive scarring will provide poor wound beds for successful nonvascular bone grafting. In these situations, transfer of healthy vascularized tissue is essential for successful reconstruction. Complication rates for mandibular reconstruction have shown a tendency to increase with the increasing soft tissue resection. Loss of a significant volume of tissue precludes immediate nonvascular bone grafting in most situations. Failure rates with immediate nonvascular bone grafting techniques have been reported as high as 50% in such situations. Therefore, when a segmental defect is associated with extensive loss of soft tissues, immediate reconstruction with vascularized tissue transfer or delayed reconstruction should be considered.
Diagnostic Studies
Diagnostic studies to assess the ability of the patient to tolerate the proposed operation or the extent of malignant disease are beyond the scope of this chapter. Diagnostic studies pertinent to mandibular reconstruction include preoperative imaging of the mandible, computer-generated models or surgical guides (tactile medical imaging), and preoperative vascular studies specific to various reconstructive techniques.
Preoperative computed tomography (CT) of the mandible is critical to establishing the anticipated defect. Plain radiographs may be useful adjuncts to clinical evaluation but lack sufficient detail alone to establish the extent of disease. CT imaging allows evaluation of disease within the marrow space and can better evaluate cortical erosions that may otherwise be missed with plain radiographs. Axial and coronal scans are the minimal requirement and may be combined with sagittal images or three-dimensional reconstructions as needed to further define the extent of tumor involvement. Magnetic resonance imaging (MRI) may be useful as an additional study to identify extension along the inferior alveolar for certain tumor biologies. Because of its poor detail of osseous anatomy, MRI is not routinely utilized alone.
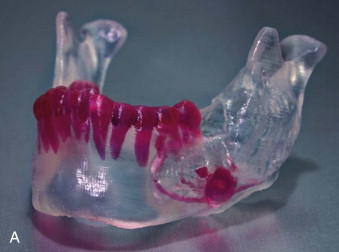
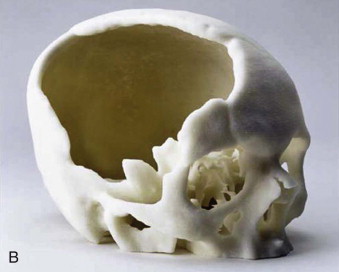
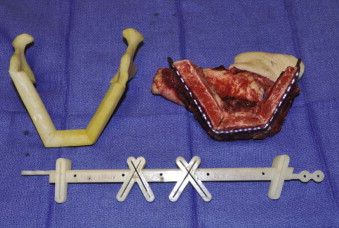
Tactile medical imaging is the display, in physical form, of anatomic data derived from medical imaging studies. Stereolithography and 3D printing represent the two main technologies for tactile medical models. Stereolithography utilizes laser photopolymerization of a liquid polymer to create an accurate, durable, translucent and sterilizable model of the patient’s skeletal anatomy ( Fig. 60-1, A ), whereas 3-D printing utilizes an inkjet-based printing method to create a skeletal model by injection of liquid into a plaster powder ( Fig. 60-1, B ). Three-dimensional printing models are accurate and opaque, but they are fragile and not sterilizable. Both allow hands-on study and treatment planning, but the stereolithographic models may also be used intraoperatively to assist with prebending of surgical fixation systems, model surgery, and shaping of bone grafts ( Fig. 60-2 ).
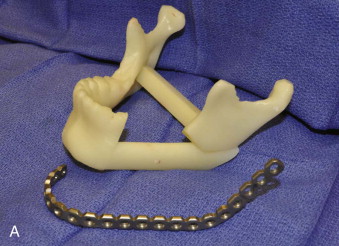
Current technology also allows for virtual treatment planning and surgery for the creation of surgical guides from computer-based images ( Fig. 60-3 ). Intraoperative use of these guides has been shown to improve accuracy of osteotomies and decrease operative time.
Preoperative vascular studies are at times necessary to delineate the recipient (neck) or donor (flap) vascular anatomy prior to free tissue transfer. The head and neck cancer patient may have unclear vascular anatomy to serve as recipient vessels for free tissue transfer, especially in the multiply operated neck. Preoperative angiogram of the head and neck can identify the presence or lack thereof of arterial structures suitable for microsurgery and can also identify the status of the internal jugular vein. Patients with vessel-depleted necks may require vein grafting or creation of a vascular loop from distant vessels. This information can be critical in the decision process and preparation in such patients and ultimately make the difference in a successful reconstruction. Preoperative assessment of donor vessels is helpful in certain situations as well. Some patients may demonstrate surgical scars from prior surgery that may have disrupted the potential donor vessels. An example would be patients with lateral thoracotomy scars or low transverse abdominal scars that are being considered for latissimus dorsi or rectus abdominis muscle transfer respectively. Preoperative angiography can confirm the patency of the donor vessels. Probably the most common use of preoperative angiography of donor vessels is associated with fibula transfer. Two clinical situations that are critical to identify in patients considered for fibula transfer are the presence of inadequate vascularity of the leg to allow harvest of the peroneal artery and the presence of a dominant peroneal artery system. Both conditions can result in ischemia of the foot and are contraindications to the harvest of the fibular flap. Studies have demonstrated that dominant peroneal systems are present in approximately 5-10% of the population. Peripheral vascular disease resulting in compromised vascularity of the lower extremity is common in the elderly, tobacco-using population characteristic of head and neck cancer patients. Screening of potential candidates with lower extremity pulse exams can identify patients needing preoperative lower extremity angiography. Patients with normal lower extremity pulse exams, in general, do not require preoperative angiography.
Treatment/Reconstructive Goals
The goal of mandibular reconstruction is the restoration of form and function of the mandibular apparatus. Specifically, this requires reestablishing an acceptable appearance and mandibular continuity, maintaining a pain-free mandibular range of motion and relatively normal soft tissue relationships within the oral cavity to facilitate speech and deglutination, and providing a foundation on which to reconstruct the dentition.
Treatment options for reconstruction of segmental defects of the mandible include collapse of the defect, mandibular reconstruction plates (MRP) with or without soft tissue flaps (no osseous reconstruction), nonvascularized bone grafts, and vascularized bone flaps. Choice of technique is influenced by several factors including (1) surgeon training/preference, (2) location of defect, (3) length of bony defect, (4) extent of oral mucosal or facial skin loss, (5) quality/vascularity of the wound bed, and (6) patient medical comorbidities.
Timing of Reconstruction
The ideal timing of mandibular reconstruction has been widely debated, especially in patients with malignant disease. Historically, proponents of a delayed or staged approach advocated a period of observation to monitor the patient for development of recurrent disease or to establish histologically clear bony margins prior to reconstruction. Today, however, it is widely accepted that immediate reconstruction may be performed without risk for a delayed diagnosis of recurrent disease. Prior to microsurgical techniques, delayed reconstruction was critical to allow maturation of the wound bed for nonvascular bone grafting. Lawson et al. reported their results with immediate and delayed reconstruction utilizing corticocancellous grafts. They reported a 90% success rate with delayed reconstruction, compared to 46% with immediate reconstruction when using nonvascularized bone grafts. Subsequent reports have demonstrated successful use of delayed, nonvascular bone grafts in select patients, and indeed nonvascular grafts are still frequently utilized today. There are, however, strong arguments for the utilization of immediate vascularized bone flaps for most segmental mandibular defects compared to delayed reconstruction with nonvascular bone grafts. These include both patient factors and treatment/technical factors. The literature supports that delayed reconstruction of segmental defects of the mandible requires temporary stabilization of the mandibular remnants with mandibular reconstruction plates (MRP) with or without soft tissue flaps; temporary stabilization with MRP/myocutaneous flaps is associated with high complication rates especially in the anterior region; MRP failures can occur within 18 months with mean time to failure reported as early as 6 to 8 months; and complication rates for nonvascular bone grafts are greater for defects larger than 6 cm compared to vascularized bone flaps.
Immediate reconstruction has several other advantages over delayed reconstruction for mandibular reconstruction. Health-related quality of life (QOL) studies have demonstrated that immediate reconstruction significantly improves QOL and that most patients prefer immediate reconstruction. Furthermore, Boyd reported that patients who underwent reconstruction with vascularized bone flaps experienced an average of 4 days life lost for secondary procedures, compared to 35 days for patients who underwent plate and soft tissue flaps.
Surgeon Training/Preference
The choice of any surgical procedure is affected by the training and skill level of the surgeon. In every specialty of medicine, new techniques or improvements of existing techniques are occurring at rapid pace. A thorough understanding of the available literature regarding techniques, complication rates, and indications and contraindications should guide the surgeon through the choice of available procedures. Although every surgeon may not be comfortable or adept with every technique, it is the responsibility of every surgeon to be familiar with the most current therapies and seek consultation or referral when in the best interest of the patient.
Collapse the Defect
The simplest technique for mandibular reconstruction is actually no reconstruction at all. Collapse of the mandibular segments and wound closure is rarely performed today but is acceptable for some patients with defects of the ramus-condyle and lateral body units. A characteristic deformity is created with deviation of the chin to the affected side and elevation of the tongue on the affected side. With appropriate physical therapy, reasonable mandibular function is maintained. The effect on speech and swallowing depends on the amount of soft tissue resected and the relationship of the remaining intraoral soft tissues following wound closure. Collapse of the defect should be reserved for select patients with significant medical comorbidities or extremely poor prognosis.
Location of Defect and Mandibular Reconstruction Plates (Mrps)
As previously mentioned, the location of the segmental defect affects the complication rate of the technique. This is most important when considering reconstruction with a MRP with or without a soft tissue flap or a delayed, nonvascularized osseous reconstruction where the patient will require temporary stabilization with a MRP. In these situations, reconstruction of defects of the anterior region demonstrates high complication rates.
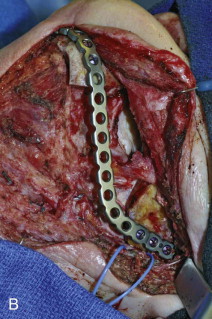
Spiessel in 1976 was the first to report bridging a tumor defect with a reconstruction plate. However, Schmoker was the first to propose reconstruction of mandibular defects with a reconstruction plate without osseous reconstruction. This concept had many early advocates through the 1980s because of its ease, lack of a second surgical donor site, the quickness for which it could be performed, and a concern for high recurrence rates. By the 1990s, many authors reported high complication rates ( Fig. 60-4 ) with MRPs, especially in the anterior region. Kim and Donoff reported their experience with 41 plates in 37 patients. They divided the study into three groups based on location of the defect. Defects of the anterior mandible had a 52% failure rate compared to 12.5% for the body segment and 7.7% for the condyle-ramus unit. The overall wound dehiscence rate was 17%. Other authors reported high complication rates for lateral defects and overall outcome. To combat the high rate of extrusion, several authors proposed the use of soft tissue flaps in conjunction with MRPs. Cordieri and Hildalgo reported their experience with soft tissue coverage of mandibular reconstruction plates for mandibular reconstruction in 14 patients; 9 patients underwent pectoralis muscle flaps and 5 underwent soft tissue free flaps for defects of the anterior mandible. The pectoralis group had a 44% extrusion rate, compared to 0% for the free flap group. They hypothesized the difference was due to tethering and tension on the pectoralis flap caused by the limitations of its reach by the nature of its pedicle attachment. Wei and colleagues reported their experience with reconstruction of composite segmental defects of the mandible utilizing MRPs and soft tissue flaps in 80 patients. The overall complication rate was 69%, with plate exposure occurring in 46%. Seventy-eight percent underwent a second reconstructive procedure. Extrusion rates have also been shown to be higher in irradiated tissues. In the author’s experience, reconstructions of anterior defects and larger lateral defects with soft tissue flaps (free or pedicled) have high rates of extrusion, especially in the irradiated patient. This is likely due to the narrow profile of the plates providing poor support of the soft tissues, in association with contracture of the soft tissue envelope creating pressure necrosis. MRPs with or without soft flaps are doomed to failure in the anterior region. Reconstruction of the mandible with MRPs or temporization with MRPs in anticipation of delayed nonvascular bone grafts should be reserved for smaller defects of the lateral or posterior units in patients not requiring radiotherapy and considered poor candidates for vascularized bone flaps.
Anterior Defects
Defects in this region represent the most difficult to reconstruct for many reasons. The symphysis has multiple forces acting across the region depending on the mandibular function. Both compressive and tensile forces are present as well as torsional forces placing significant stress on any construct. The curved shape of the symphysis tends to be more difficult to re-create compared to the relative straight segments of the posterior and lateral regions. Its shape may influence the appearance of the lip and vertical height of the lower face. The symphysis also serves as the site for attachment of the suprahyoid and tongue musculature. When nonosseous reconstructions are performed in this region, the force of these muscle attachments is essentially conferred to the soft tissue envelope. This results in increased pressure of the soft tissues against the reconstruction plates and the opportunity for pressure necrosis of the overlying tissue with subsequent hardware exposure. As previously mentioned, anterior defects reconstructed with reconstruction plates and soft tissue flaps have been shown to be at high risk of failure. Although vascularized bone flaps may have some limitations in contouring, the high failure rates associated with MRPs in the anterior region (without immediate osseous reconstruction) make them the procedure of choice for the anterior region.
Lateral Defects
Lateral defects characteristically are less complicated to reconstruct. They are relatively straight, do not have as significant muscle attachments, demonstrate lower complication rates compared to anterior defects, and in general have less impact on facial form. Lateral defects smaller than 6 cm and associated with benign disease are candidates for nonvascular bone grafting in patients not desiring immediate reconstruction. Lateral defects 6 cm and larger, associated with larger soft tissue loss, and having a history or need for radiation therapy should be reconstructed with vascularized bone flaps. Two important considerations present themselves when planning reconstruction with dental rehabilitation of lateral defects: the lateral and vertical position of the osseous construct. The normal mandibular body contour tends to be wider than the position of the alveolar bone. Therefore, when planning the contour of reconstruction plates and the position of the bone graft or flap there is often a balance between placing the bone in a location to recreate the ideal facial contour versus a location that is ideal or at least usable for dental implants. Vertical position of bone flaps must also be considered. Fibula bone flaps have a much lower profile compared to the dentate mandible. Setting the reconstruction plate and the bone flap at the inferior border will recreate the most ideal jawline but may create a significant prosthetic challenge when one considers the location of the bone in relation to the occlusal platform. In the edentulous mandible, the fibula is an excellent size match and vertical height does not usually pose a problem.
Posterior Defects
Posterior defects, similar to lateral defects, involve a relatively straight segment of the mandible and are therefore less complicated to reconstruct unless involving the condylar process. They can impact facial form, including posterior vertical height of the mandible, and may create a more significant impact on mandibular range of motion because of the relationship with the temporomandibular joint and muscles of mastication. Posterior defects do represent a region that is amenable to allowing collapse of the defect without reconstruction when dictated by the patient’s clinical situation. Depending on local and patient factors such as volume of tissue loss, disease process, status of the temporomandibular joint, and radiation therapy defects of the posterior unit may be reconstructed with costochondral/corticancellous grafts, alloplastic prostheses, or vascular bone flaps. Alloplastic joint prostheses probably do not have a role for true segmental defects involving the posterior unit and should be reserved for isolated loss of the condylar process in the absence of radiation therapy and significant tissue loss. Marx and colleagues reported the successful use of reconstruction plates with condylar prostheses for defects of the posterior unit including the condylar process. Complications including plate fracture and erosion into the glenoid fossa have been reported ( Fig. 60-5
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


