Management of head and neck cancer is a difficult but common challenge in the practice of oral and maxillofacial surgery. Radiotherapy is a proven modality for the treatment and palliation of head and neck malignancy. Despite its benefit in the treatment of cancer, radiation has an immense potential for causing acute and chronic complications. Oral and maxillofacial surgeons and other dental specialists are not directly involved in the administration of radiotherapy, but they are often responsible for the prevention and treatment of adverse effects and complications resulting from it. Some of these complications are temporary and may resolve with time, and these are easy to manage; however, others, such as osteoradionecrosis (ORN), can be devastating and difficult to treat.
Radiation Physiology
Radiation can be nonionizing and ionizing. Nonionizing radiation such as radio waves, microwaves, and light waves have little energy and do not have a therapeutic role in cancer treatment. Ionizing radiation has high energy. It forms ions as it passes through tissues by dislodging electrons from atoms. Ionization results in cell death or irreversible damage to DNA with consequent loss of reproductive capabilities of the affected cells. It is this feature that makes ionizing radiation effective for the treatment of malignancy not only in the head and neck area but also in general.
The two main types of ionizing radiation are electromagnetic and particulate. Electromagnetic radiation comes from photons. Photons are elementary particles that have intrinsic properties of charge, mass, and spin, depending on the circumstances. Photons originating from the atomic nucleus produce gamma rays, and photons originating from electrons around the atomic nucleus produce x-rays. Particulate radiation includes electrons, protons, neutrons, and alpha and beta particles. The radiation source can be outside the body or implanted as radioactive seeds or needles (brachytherapy). The more common types of radiation used in the head and neck are high-energy photons, electrons, protons, and neutrons. Protons are the earliest type of radiation. Protons deposit most of their radiation energy in what is known as the Bragg peak. The depth at which the Bragg peak occurs is energy-dependent and can be controlled to allow the maximum dose to be delivered to the target tumor with minimal damage to surrounding normal tissues. Proton radiation is available in just a few centers in the United States. Neutron beam radiation causes severe long-term complications and is used rarely for tumors resistant to other types of radiation. Intensity-modulated radiation therapy (IMRT) is a protocol in which multiple beams of radiation are delivered at different angles and intensities to target a tumor while minimizing radiation injury to the surrounding tissues. This technique requires tridimensional mapping of the tumor and careful positioning and immobilization of the patient during treatment.
The amount of radiation delivered to a target area is the radiation dose. The unit for measuring radiation is the gray (Gy). The amount of radiation in an abdominal radiograph is 1.4 milligray (mGy). The typical radiation dose for the treatment of malignancies in the head and neck region ranges from 5000 to 7600 centigray (cGy) and averages around 7000 cGy. The total dose is delivered in fractions, usually 200 cGy daily. Considerable research has been performed to define the most appropriate fractionation schedule. Variations in protocol, called hypofractionation and hyperfractionation, deliver fewer but larger doses or smaller, more frequent doses. Fractionation is an important concept in radiotherapy. It provides normal tissue in the irradiated field the opportunity to heal between doses, thereby decreasing the amount of late complications. The presence of oxygen at the time of irradiation significantly enhances the effects of ionizing radiation in living tissues. Tumors often have areas of hypoxia since uncontrolled rapid growth can compress blood vessels and reduce blood flow. Because of the high number of cells, tumors often outgrow their blood supply, which also results in areas of hypoxia. Fractionation reduces the number of cells and the size of the tumor and allows reoxygenation, which directly increases the efficacy of radiation. Other variables affecting the sensitivity of cells to radiation include the type of cell and its stage in the reproductive cycle. Germinal and lymphoreticular cells are the most sensitive. Endothelial cells and fibroblasts have intermediate sensitivity. However, even reduced damage to these cell types may diminish the healing potential of tissues and result in late complications. Finally, muscle and nerve cells are the most resistant to the effects of ionizing radiation. Because tumor cells replicate at a rate faster than normal, they are more likely to be irradiated at a vulnerable time in their cell cycle, and they therefore are the most sensitive to radiation therapy.
Side Effects of Radiation Therapy
The goal of radiotherapy in cancer treatment is eradication of every viable tumor cell with minimal damage to normal tissue. Unfortunately, it is not unexpected for cancer patients undergoing radiotherapy to suffer side effects resulting from damage to healthy tissue in the path of the radiation. The oral cavity and pharynx are covered with specialized mucosa that has rapid epithelial turnover and a rich vascularity to support it. The mandibular alveolus has a rapid bone cell turnover rate that can be 10 times that of long bones. These features make this tissue susceptible to radiation-induced damage. Adverse effects can occur acutely or late. Acute adverse effects can cause significant discomfort to the patient. However, they are short term and generally resolve within weeks after completion of radiation therapy. The goal in management of these conditions is to provide immediate pain relief. Late adverse effects, such as ORN, xerostomia, and radiation-induced caries, cause significant morbidly and are much more difficult to treat. They also have a greater impact on quality of life and the distress level of cancer patients.
Radiation Mucositis
Radiation-induced mucositis occurs in almost all patients undergoing radiotherapy for the treatment of head and neck cancer. It is the result of repeated radiation insult to otherwise normal mucosa and usually develops within the first 2 weeks of treatment. The adverse clinical signs are evident by the second or third week. The early stage is characterized by a whitish discoloration caused by the lack of sufficient desquamation of keratin. This evolves into a diffuse erythema with the presence of fibrinous exudates ( Fig. 28-1 ). The mucosa becomes friable and often ulcerated. Radiation-induced mucositis is a debilitating condition that produces a state of general discomfort. Burning pain, dysphagia, weight loss, and speech difficulties are some of the common complaints. Rarely, radiation oncologists will agree to interrupt the treatment because of side effects; however, ulcerative mucositis is the major limitation to continuous, uninterrupted radiotherapy.
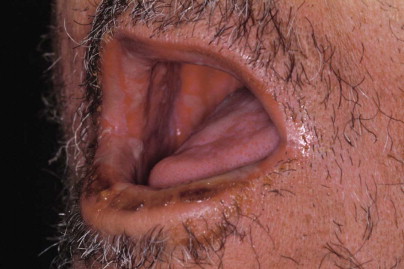
Radiation-induced mucositis usually resolves within 3 to 4 weeks after completion of the last fraction. The treatment goals for these patients are prevention of opportunistic infection and pain control. It has been reported that 76% of head and neck cancer patients are found to be noncompliant with routine dental care and oral hygiene in pre-radiation consultation. Ironically, adequate oral hygiene is a key factor in the prevention of infection resulting from bacterial colonization of ulcerated tissues. Traditional hygiene routines of tooth brushing and dental floss can be extremely painful. Therefore, antibiotic oral rinses play an important role in intraoral bacterial control. Chlorhexidine is a broad-spectrum topical antimicrobial agent that is widely prescribed by oral surgeons, other dental specialists, and general dentists. Use of 0.12% chlorhexidine oral rinse is recommended in patients with mild to severe radiation-induced mucositis. Use of a chlorhexidine-soaked foam brush is an alternative for maintaining oral hygiene. Viscous lidocaine 2% gel is usually helpful in controlling pain. Systemic analgesics, including nonsteroidal anti-inflammatory drugs and narcotics, are concomitantly prescribed when necessary. Antibiotics are indicated only in patients with secondary infection who have systemic manifestations such as fever or lymphadenopathy.
Radiation Dermatitis
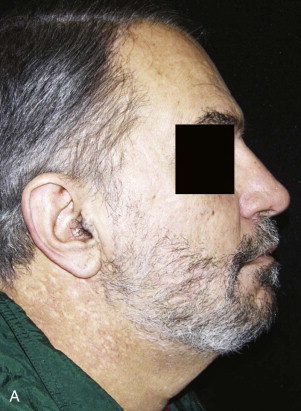
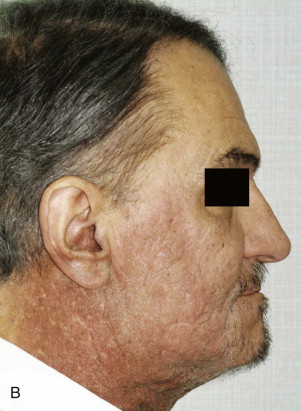
Radiation-induced dermatitis usually occurs within 90 days of starting treatment. Many of the skin changes are minor and reversible. The initial changes may be undetectable and can occur hours after exposure to radiation and fade within hours to days. Apparent and sustained changes lasting for up to 2 weeks after completion of radiation therapy are most likely mediated by cytokines. They are described as a blanchable reactive pink hue with no other epidermal changes. Cutaneous changes may be classified as acute, late, or chronic. The standard for evaluation of radiation-induced dermatitis is the National Cancer Institute’s common toxicity criteria version 3.0. Grade 1 changes include mild or generalized erythema with pruritus, epilation, and depigmentation. Grade 2 changes occur after radiation doses higher than 4000 cGy. At this stage persistent erythema may progress to loss of the epidermis limited to skin folds. This is more common in breast cancer patients because of the more pronounced skin folds. These changes usually peak 1 to 2 weeks after completing radiation therapy. Epidermal regeneration is noted within 3 to 5 weeks, with complete healing taking place in 1 to 3 months. When moist desquamation extends beyond the skin fold, it is classified as grade 3. Ulcer development, hemorrhage, and necrosis are considered grade 4 changes. As with radiation-induced mucositis, secondary infection is a common complication and thus skin care and hygiene are extremely important. Chronic radiation-induced dermatitis can appear months to years after radiotherapy. Manifestations include the following: textural changes, hyperpigmentation, hypopigmentation, telangiectasia, and fibrosis. Alopecia and decreased or absent sweating result from damage to hair follicles and sebaceous glands ( Fig. 28-2 ). Some of these changes may improve and eventually normalize. Depending on variables such as the total dose, location, severity of the initial injury, skin type, and individual patient co-morbid conditions, they can be more severe or permanent. The scalp is more tolerant of the effects of radiation than the face, neck, or chest are. The treatment goals are pain control, minimization of transepidermal water loss, and prevention of infection. Progression of erythema and dry desquamation to moist desquamation should be prevented. Petrolatum-base emollients are commonly prescribed for the treatment of radiation-induced dermatitis. Non–petrolatum-based products with castor oil, balsam of Peru, trypsin, or aloe vera are also used. Although the indication for topical steroids is controversial, they have likewise been used for the prevention and treatment of this condition. Pain control is achieved with oral analgesics. Topical and systemic antibiotics are indicated in the event of cutaneous infection.
Xerostomia
A healthy adult produces about 1.5 liters of saliva per day. Saliva lubricates the oral mucosa and pharynx, facilitates speech and swallowing, and is involved in the control of oral flora. Saliva also aids in the digestion of food and buffering of acids produced by the fermentation of carbohydrates. Radiation-induced xerostomia is a serious condition that significantly affects the quality of life of head and neck cancer patients. The slow turnover rate of glandular epithelium may provide salivary glands some degree of radio resistance. However, the ultimately decreased perfusion and destruction of the microvasculature often result in irreversible damage. In addition to the normal complaint of dry mouth, xerostomia has multiple associated sequelae. Dysphagia, dysgeusia, dysosmia, pain, mucositis, and chewing and speech difficulty are some of the common complaints of patients with this condition. Decreased or absent salivary flow promotes the proliferation of cariogenic bacteria such as Streptococcus mutans and Lactobacillus species. Caries and periodontal disease can be directly related to radiation. The changes in oral flora are responsible for these conditions outside the field of radiation. Radiation doses higher than 3000 to 3500 cGy are associated with an increased risk for significant salivary gland dysfunction. Doses lower than 2000 cGy may produce reversible damage. Marked irreversible and degenerative changes in acinar cells and glandular ductal epithelium occur with radiation doses higher than 5000 cGy.
Reduced salivary flow is seen in the first week of radiation therapy and may decrease to about 20% of baseline after 7 weeks. Partial recovery may be possible within 12 to 18 months after treatment. The use of IMRT protocols can reduce the radiation dose delivered to the salivary glands and allow preservation of some gland function. Two pharmaceutical agents have been advocated for the protection of salivary glands during treatment in an effort to minimize radiation injury. Pilocarpine (Salagen, MGI Pharma, Minneapolis, Minn) is a sialagogue that promotes salivation by stimulating muscarinic receptors on the salivary glands. Studies reported that a dose of 5 mg three to five times a day during radiation therapy did not prove to be effective in the preservation of salivary gland function. The second agent is amifostine (Ethyol, MedImmune Oncology, Inc., Gaithersburg, Md). This drug has been approved by the U.S. Food and Drug Administration (FDA) for reduction of radiation-induced xerostomia. It is a sulfhydryl compound that has the ability to donate hydrogen ions to radiation-generated free oxygen radicals and thereby prevent oxidation damage. Amifostine has been shown to significantly reduce the incidence of acute and chronic radiation-induced xerostomia. Furthermore it appears to offer selective protection to normal cells without affecting the efficacy of radiation in tumor treatment. Amifostine is given daily 15 to 30 minutes before radiation therapy at a dose of 200 mg/m 2 . Reported side effects are nausea, vomiting, hypotension, and local skin reaction at the injection site. Another reported technique for prevention of xerostomia is surgical transfer of the submandibular gland to the submental space to place it outside the radiation field.
It is common to see patients suffering from radiation-induced xerostomia with water bottles at all times. They require constant sipping to maintain the moisture of the oral mucosa. Salivary substitutes function as a temporary relief of discomfort by introducing moisture into the oral cavity ; however, they do not replace the antibacterial and immunologic protection of natural saliva.
This population of patients also requires comprehensive dental care and close follow-up. Oral hygiene is a key factor in the prevention of caries and periodontal disease. Antimicrobial rinses (0.12% chlorhexidine) and topical fluoride application are recommended. Pilocarpine is approved by the FDA as a sialogogue agent; it is prescribed three times a day in doses of 5 to 10 mg. Increased salivary secretion occurs within 30 minutes after ingestion. Treatment lasts for 8 to 12 weeks, and it may be used for longer periods as maintenance therapy. Bethanechol (Urecholine, Odyssey Pharmaceuticals, East Hanover, NJ) is also used for the systemic treatment of xerostomia. It is given in doses of 25 to 50 mg three times a day. It has been suggested that the use of sugarless gum and candy may stimulate residual gland function. Sialogogue agents will increase salivary secretion by stimulating muscarinic receptors in viable residual gland tissue. Consequently, they are not effective when no salivary gland tissue survives radiation therapy.
Trismus
The cause of trismus in head and neck cancer patients is multifactorial. Limitation of mandibular range of motion can be a consequence of invasion of tumor into the masticatory muscles or the temporomandibular joint (TMJ), mechanical obstruction of the coronoid process by tumor growth, and scarring secondary to surgery. Trismus is also seen in conjunction with ORN and usually improves after definitive treatment. Radiation can also be directly associated with injury to tissues. Radiation-induced trismus has a reported incidence of 6% to 86%. This wide range is explained by the multiple interpretations of how much limitation represents trismus. Other variables such as total radiation dose, type of radiation, location of the primary tumor, surgical procedures performed, and other individual patient factors can influence the incidence of trismus. It is very important for the clinician to identify the etiology before implementing therapy. Radiation-induced trismus is directly related to radiation damage to the masticatory muscles, TMJ, oral mucosa, and facial skin. Fibrosis of the oral mucosa, especially at the pterygomandibular raphe, anterior tonsillar pillar, and retromolar areas, can significantly restrict mandibular range of motion. Trismus may compromise oral intake, speech, the ability to secure the airway (and thus increase the risk for aspiration), dental hygiene, and dental treatment. Oromandibular dystonia, which is characterized by painful muscle contractions, cramps, and pain, has also been reported but is rare when compared with radiation-induced trismus. Pain is reported in 15% to 30% of patients after radiation therapy. It has been suggested that radiation doses greater than 5000 cGy to the TMJ and masticatory muscles are required to induce trismus. The onset of trismus is usually seen early in the postradiation period, but it can develop 3 to 6 months after completion of therapy. The condition is progressive and can worsen with time for up to 9 months after onset. Botulinum toxin A applied to each masseter muscle in a dose of 50 units has shown some value in the treatment of pain and oromandibular dystonia. However, it was ineffective in treating trismus. Physical therapy (PT) with manual stretching exercises, tongue depressors, or mechanical devices such as the Therabite (Altos Medical, West Allis, Wisc) is a valuable tool in prevention and treatment of trismus. To be effective, PT must be initiated early after completion of radiation therapy and requires that patient compliance be maintained for several months afterward.
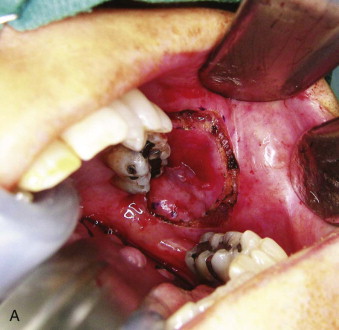
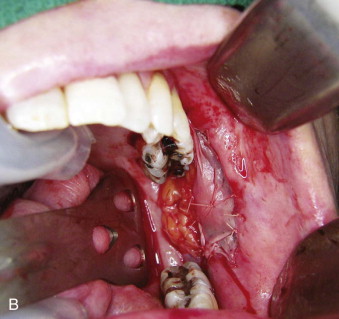
Forced mouth-opening under general anesthesia is also used; however, it is less predictable and associated with increased risk for dentoalveolar and soft tissue trauma. Some surgical options are available for patients with severe trismus or those who are not responsive to PT. If the trismus is due to scarring or fibrosis of the oral mucosa, excision of the specific affected tissues and grafting with a myocutaneous or free microvascular flap can be performed ( Fig. 28-3 ). When the fibrosis is located at the masticatory muscles, surgical excision of tissue can compromise the blood supply to the mandible and induce ORN. Coronoidectomy can significantly improve mandibular range of motion. The maximum interincisal opening 6 months to 1 year after surgery is usually less than what is initially obtained in the operating room or immediately postoperatively. The use of adjutant PT at home helps maintain the results long-term.
Dysphagia
Swallowing is a complex process that requires precise coordination of several muscle groups in the oral cavity, pharynx, larynx, and esophagus. Normal swallowing has involuntary and voluntary components and is divided into four phases: oral preparation, oral, pharyngeal, and esophageal. Dysphagia in the setting of head and neck cancer can be caused by tissue loss and scarring secondary to surgical excision and loss of sensation as a result of transection of nerves and muscles. It can also be related to the size and location of the primary tumor. Many of the common sequelae of radiation therapy, such as trismus, rampant caries, xerostomia, and mucositis, can individually or in conjunction considerably affect swallowing. Dysphagia is often accompanied by odynophagia. It also appears as a late complication that is directly related to radiation damage to the structures involved in swallowing. Lymphedema, fibrosis and decreased contractility of the muscles of mastication, and loss of coordination and muscle strength, especially of the pharyngeal constrictors, adversely affect the harmony of the swallowing cycle. Hypopharyngeal and upper esophageal strictures are not uncommon and may require multiple dilation procedures to improve swallowing. The use of feeding tubes for prolonged periods may result in muscular atrophy with subsequent dysphagia.
Dysphagia increases the risk for aspiration. The cough reflex is usually ineffective or non-inexistent in almost half of affected patients. Silent aspiration may result in aspiration pneumonia as a post-treatment complication of radiation therapy. Patients with tracheostomy tubes may also experience dysphagia and increased risk for aspiration. The most common methods for evaluation of swallowing function are the flexible endoscopic evaluation of swallowing (FEES) and the modified barium swallowing study (MBSS). FEES can easily be performed at bedside or in the office setting; however, it is useful only for evaluation of the pharyngeal phase of swallowing. Thus, many clinicians prefer MBSS over FEES.
Dysphagia and radiation have a dose-dependent relationship ; therefore, modifications in the treatment protocols aimed at reducing the dose given to healthy tissues may decrease the risk for and severity of dysphagia. Use of IMRT can reduce radiation injury not only to the pharyngeal musculature but also to the salivary glands. Use of amifostine in the regimen previously described in an attempt to decrease salivary gland dysfunction is also valuable in the prevention of dysphagia.
The greatest incidence of swallowing disorders is seen 3 months after treatment. Significant improvement generally occurs in the following 12 months. When required, rehabilitation is usually provided by the speech-language pathologist and consists of postural techniques, sensory techniques, motor exercises, swallowing maneuvers, and changes in diet. The majority of patients recover swallowing function over time without rehabilitation. It has been suggested that early intervention is superior to delayed intervention.
Radiation Caries
Radiation-induced caries can be a direct consequence of radiation damage to the tooth structure or indirect damage as a result of xerostomia, changes in oral flora, deficient oral hygiene, and dietary changes. Salivary gland dysfunction with a consequent decrease in salivary flow has a dramatic impact on dental health. Decreases in secretory immunoglobulin A, changes in pH, decreased bicarbonate concentration with reduced buffering capacity, and changes in the oral flora have been reported in patients with radiation-induced xerostomia. These changes are responsible for radiation-induced caries outside the field of radiation. Direct radiation damage to the teeth is due to pulpal necrosis and dentinal dehydration. Radiation-induced caries usually occurs at the gingival margin, cusp tips, incisal borders, and dentine-enamel junction. It is manifested as hard black areas and resembles dentinogenesis imperfecta.
It is important for the clinician to differentiate the concept of direct and indirect radiation-induced caries. Oral hygiene, prophylaxis, and topical fluoride may be effective in the treatment of indirect radiation-induced caries; however, they have no value in treating direct radiation-induced caries because it is a direct consequence of radiation damage to the tooth structure and is not preventable. This mandates that any teeth planned to be or that were in the path of a significant radiation dose should be extracted before radiation therapy or early after it. The suggested high-risk dose for direct radiation-induced caries is 6000 cGy or higher. It has been reported that 76% of head and neck cancer patients are noncompliant with dental care and oral hygiene. Maintenance of adequate oral hygiene during and after radiation therapy can be extremely difficult and painful because of the mucositis, dermatitis, and trismus. Many of these patients have preexisting dental disease and will simultaneously benefit from multiple extractions despite the fact that they are scheduled for radiation therapy. Performing heroic dentistry to restore and save teeth in these cases will only delay extractions and increase the risk for ORN. Maintenance of oral hygiene is a key factor in the prevention of indirect radiation-induced caries. Periodic dental evaluation and treatment and topical fluoride application are usually effective and recommended.
Malnutrition
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


