▪
HISTORICAL PERSPECTIVE ON LOCAL ANESTHESIA
The efforts of human kind to find the means to control pain presents as one of the greatest challenges in medicine. Pain is a phenomenon wisely instituted by nature as a warning sign of a condition that may be detrimental to our bodies. From the earliest antiquities down through the long centuries, humans have resorted to many measures including superstition in an effort to blunt this noxious stimulus. Yet thankfully as our knowledge of the human body grew, so did our understanding of pain. As Hippocrates once stated, “ Divinium est opus sedare dolorem ”—divine is the work to subdue pain.
Some of the earliest references to the use of pain-reducing compounds were found in Homer’s “Odyssey,” when Helen gave Ulysses and his comrades the “sorrow easing drug,” which consisted of a mixture of poppy and Indian hemp. During the siege of Troy, the Greeks used anodyne and astringents to ease the pain of their wounds, completely unaware of its antiseptic property. It was through trial and error that primitive man used cold to lessen the pain and in time learned that pressure on the affected area had a more pronounced effect. In the early times, the Assyrians applied pressure over the carotid to cut off the blood supply to the brain, thus producing a transient syncopal-like episode, to obtain a certain degree of anesthesia during circumcisions. This may explain why the literal Greek and Russian translation of carotid artery is “the artery of sleep.” In the year 50 ad , Pedanius Dioscorides is said to have made the first attempt to produce an anesthetic paste, allowing it to act as topical anesthetic. By pulverizing Memphis stone and mixing it with vinegar, the resultant carbonic acid produced a cold stimulus, causing anesthesia over the affected area.
For reasons unknown, the middle ages of humankind did not present with any new discoveries or advances in local anesthesia. It was not until the nineteenth century that the literature began to first describe a chemical with some anesthetic properties. Supposedly, dating back to 1532, the Indians in the highlands of Peru chewed the leaves of coca shrub to relieve fatigue and hunger and to produce a feeling of exhilaration. Carl Scherzir in 1856 reported the anesthetic properties of the coca leaf. In 1859 a German chemist, Albert Neimann, was given credit for being the first to extract cocaine in its pure form. This was despite the fact that a year earlier another German chemist by the name of Friedrich Godeke had also isolated the active ingredient, but had called it “erythroxylin.” It was not until 1865 that one of Niemann’s disciples, Wilhelm Lossen, finally determined the correct formulation of cocaine as C 17 H 21 NO 4 .
In the mid-1860s, as anesthesia began to receive more attention, Sir Benjamin Ward Richardson demonstrated the use of ether spray to anesthetize skin. Around the same time a young Viennese physician, Sigmund Freud, became interested in cocaine’s effect on mood and the psyche. He subsequently administered it to a colleague, Ernst Fleischl Von-Marxow, in an effort to free him of his morphine dependence after a thumb amputation. Ironically, Freud’s colleague was able to overcome his addiction, but he himself began to experiment with cocaine and noticed that it removed his depression. Subsequently, Freud became addicted to cocaine, which took him 10 years to overcome. It was during the same time that Carl Koller, then an ophthalmology resident at the University of Vienna, began working with Freud in his physiology lab to perform experiments using cocaine. Koller, who had read that cocaine made the tongue go numb, decided to try it on the conjunctiva. The rest is history, as he became aware of cocaine’s anesthetic and mydriatic effect. He was successfully able to demonstrate cocaine’s activity on various animal species and even himself. Then in 1884, at the Congress of Ophthalmologists held in Heidelberg, Germany, Koller’s findings were read at the conference, propagating the properties of cocaine.
Within a 12-month period, the newly discovered properties of this drug were used in every important clinic in the world. Many were thrust into using cocaine, without any regard for its potent side effects, leading to many fatalities. By one account between 1884 and 1891, 200 cases of systemic intoxication and 13 deaths were reported. It was not until Reclus and Schleich’s introduction of “infiltrative anesthesia” that a drop in fatalities was noted. As news of cocaine spread around the world, some in the United States began to experiment with its use. In 1884 at Roosevelt Hospital in New York, Richard John Hall and William Stewart Halsted were the first to describe regional block or “perineuronal” anesthesia. Using cocaine they performed what today is referred to as infraorbital and inferior alveolar nerve blocks for dental operations and later perfected many other regional anesthetic techniques. Halsted, Hall, and co-workers held weekly demonstrations and in “ the best tradition of times, experimented on themselves and innocently paid the price of becoming habitual users .” It took him 2 years to overcome his addiction.
By the1890s, the adverse effects of cocaine had been realized, leading to a more cautious approach in its use. As known today, these side effects include: cardiac stimulation, peripheral vasoconstriction, and the excitation of the central nervous system (CNS) along with physical and psychological dependence. The hyperexcitation of the cardiovascular system was later found to be due to the blockage of norepinephrine (NE) uptake at the neural terminal end. This stimulatory effect on the cardiovascular system, combined with the vasoconstrictive effects on the coronary vasculature is now known to cause myocardial infarctions in susceptible individuals. The euphoric effects and the abuse potential of the drug is related to its ability to block both dopamine (DA) and NE reuptake at key sites within the brain. Some postulate that increased levels of DA lead to dependence. This is due to DA’s role as a part of the “reward system” in the complex neurotransmitter system of the brain.
With advances in compounding and better understanding of organic chemistry, synthetic derivatives of cocaine were developed to prevent its unfavorable side effects. The first major breakthrough was the synthesis of an ester called procaine (Novocain) by Alfred Einhorn in 1904. It was not until nearly 40 years later that this development was followed by the synthesis of lidocaine (Xylocaine), the first amide local anesthetic, by Nils Lofgren. In comparison lidocaine possessed a greater potency, with a more rapid onset and most importantly less allergenicity. As a result, lidocaine displaced procaine as the drug of choice in most clinical settings.
Currently, there are more than a dozen local anesthetics, each with a distinct set of properties and side effects. With the outside pressures of the insurance companies, third-party payers, and the need to reduce hospital stays, “day surgery” is becoming a crucial element of a surgical practice. As a result, the use of local anesthetics has become essential in providing expedient service and care to the patient. This trend has been seen in most surgical subspecialties, including oral and maxillofacial surgery. A recent survey of 865 board-certified German plastic surgeons demonstrated an increased use of local anesthetics for cosmetic surgery of the head and neck, with 1% prilocaine as the most popular local anesthetic, followed by 1% lidocaine. Unfortunately, in the same time period, adverse cardiovascular reactions are also up by 8.1%. This increased use of local anesthetics, along with the aging population and their associated comorbidities, makes it prudent for clinicians to be well versed in each type of local anesthetic and their distinct properties.
▪
CHEMISTRY OF LOCAL ANESTHETICS
It is now known that local anesthetics exert the majority of their clinical actions through the blockage of nerve impulses by inhibiting the normal function of voltage-sensitive Na + channels. This in turn will prevent the noxious stimuli from reaching the brain and producing the sensation of pain. All injectable local anesthetics are composed of three structural domains: aromatic residue, intermediate chain, and amino terminus ( Figure 3-1 ). The aromatic or lipophilic portion of the molecule allows the drug to penetrate lipid-rich nerve sheaths and nerve membranes. The intermediate portion of the molecule affords the necessary spatial separation between the lipophilic and hydrophilic portions and divides local anesthetics into two distinct chemical classes: the esters (-COO-) and the amides (-NHCO-). Lastly the tertiary or second amino end provides the hydrophilic properties to the molecule. This ensures solubility of local anesthetic in the dental cartridge and the interstitial fluid after injection. By design benzocaine, which is the most commonly used topical local anesthetic, lacks this amino terminus and therefore can only be used topically (see Figure 3-1 ).
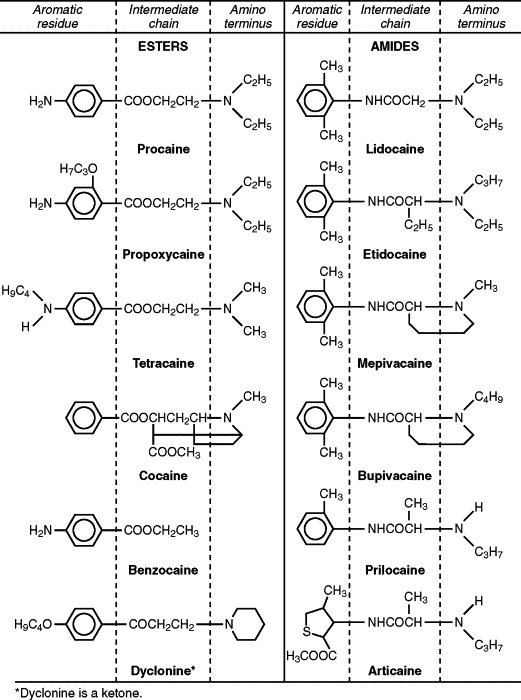
An easy way to determine whether a local anesthetic is an ester or an amide is to look at the prefix of the generic name before “-caine.” If the “I” appears in the prefix, then it is an “I”de, such as lidocaine or bupivacaine. Ester local anesthetics do not contain the letter “I,” such as benzocaine or procaine ( Table 3-1 ).
| Esters | Amides |
|---|---|
| Benzocaine | Articaine |
| Cocaine | Bupivacaine |
| Procaine | Lidocaine |
| Propoxycaine | Mepivacaine |
| Tetracaine | Prilocaine |
| Ropivacaine |
The esters, represented by drugs, such as benzocaine, cocaine, procaine, propoxycaine, and tetracaine, are metabolized primarily by plasma pseudocholinesterases. A byproduct of this metabolism is the formation of para-aminobenzoic acid (PABA), which has been implicated in the development of allergic responses in a small but significant portion of the general population. A structurally related chemical, methylparaben, was used as a preservative in amide local anesthetic solutions until it was discovered that it also produced allergic reactions in susceptible patients. Subsequently, methylparaben was removed from all dental cartridges, except in multidose vials.
Amide local anesthetics are represented by articaine, bupivacaine, lidocaine, mepivacaine, prilocaine, and etidocaine. As a result of their lower risk of allergic reactions, this class of local anesthetics has replaced the esters as the local anesthetic of choice. However, amides, which are metabolized primarily in the liver, can become problematic if they are used in patients with compromised liver function. Estimates show that by the age of 65, liver function is only 65% of normal. Therefore in healthy patients over 65, it is wise to reduce the maximum amount of local anesthetic to about half of what would be used in healthy young adults.
▪
CHEMISTRY OF LOCAL ANESTHETICS
It is now known that local anesthetics exert the majority of their clinical actions through the blockage of nerve impulses by inhibiting the normal function of voltage-sensitive Na + channels. This in turn will prevent the noxious stimuli from reaching the brain and producing the sensation of pain. All injectable local anesthetics are composed of three structural domains: aromatic residue, intermediate chain, and amino terminus ( Figure 3-1 ). The aromatic or lipophilic portion of the molecule allows the drug to penetrate lipid-rich nerve sheaths and nerve membranes. The intermediate portion of the molecule affords the necessary spatial separation between the lipophilic and hydrophilic portions and divides local anesthetics into two distinct chemical classes: the esters (-COO-) and the amides (-NHCO-). Lastly the tertiary or second amino end provides the hydrophilic properties to the molecule. This ensures solubility of local anesthetic in the dental cartridge and the interstitial fluid after injection. By design benzocaine, which is the most commonly used topical local anesthetic, lacks this amino terminus and therefore can only be used topically (see Figure 3-1 ).
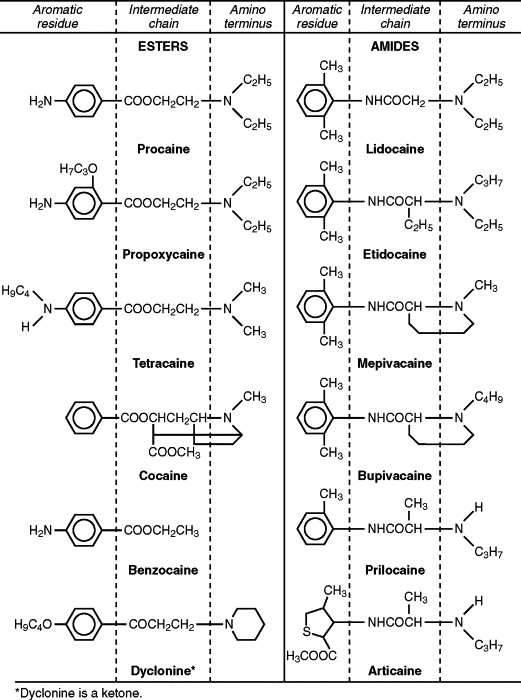
An easy way to determine whether a local anesthetic is an ester or an amide is to look at the prefix of the generic name before “-caine.” If the “I” appears in the prefix, then it is an “I”de, such as lidocaine or bupivacaine. Ester local anesthetics do not contain the letter “I,” such as benzocaine or procaine ( Table 3-1 ).
| Esters | Amides |
|---|---|
| Benzocaine | Articaine |
| Cocaine | Bupivacaine |
| Procaine | Lidocaine |
| Propoxycaine | Mepivacaine |
| Tetracaine | Prilocaine |
| Ropivacaine |
The esters, represented by drugs, such as benzocaine, cocaine, procaine, propoxycaine, and tetracaine, are metabolized primarily by plasma pseudocholinesterases. A byproduct of this metabolism is the formation of para-aminobenzoic acid (PABA), which has been implicated in the development of allergic responses in a small but significant portion of the general population. A structurally related chemical, methylparaben, was used as a preservative in amide local anesthetic solutions until it was discovered that it also produced allergic reactions in susceptible patients. Subsequently, methylparaben was removed from all dental cartridges, except in multidose vials.
Amide local anesthetics are represented by articaine, bupivacaine, lidocaine, mepivacaine, prilocaine, and etidocaine. As a result of their lower risk of allergic reactions, this class of local anesthetics has replaced the esters as the local anesthetic of choice. However, amides, which are metabolized primarily in the liver, can become problematic if they are used in patients with compromised liver function. Estimates show that by the age of 65, liver function is only 65% of normal. Therefore in healthy patients over 65, it is wise to reduce the maximum amount of local anesthetic to about half of what would be used in healthy young adults.
▪
pH EFFECTS ON LOCAL ANESTHETICS
The pharmacodynamics of local anesthetics is affected by several variables, including the pH of the solution and the surrounding soft tissue. Injectable local anesthetics are weak bases with a pKa range of 7.7 to 8.9 ( Table 3-2 ). This will cause them to exist in two forms: a free base or neutral form and cationic or positively charged form. Because a lipophilic form of local anesthetic is required for better penetration of neuronal tissue, the uncharged free base form readily penetrates neural tissue. Conversely the cationic form has a more difficult time diffusing through the membrane ( Figure 3-2 ).
| Drug | pKa | % Cationic | % Free Base | Onset Time (Min) |
|---|---|---|---|---|
| Mepivacaine | 7.7 | 67 | 33 | 2–4 |
| Lidocaine | 7.8 | 71 | 29 | 2–4 |
| Prilocaine | 7.8 | 71 | 29 | 2–4 |
| Articaine | 7.8 | 71 | 29 | 2–4 |
| Etidocaine | 7.9 | 76 | 24 | 2–4 |
| Bupivacaine | 8.1 | 83 | 17 | 5–8 |
| Propoxycaine | 8.9 | 97 | 3 | 9–14 |
| Procaine | 8.9 | 97 | 3 | 14–18 |
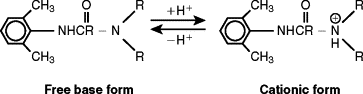
The Henderson-Hasselbalch equation for weak bases can predict what proportion of local anesthetic will exist in the two ionic states.
When the pH of the surrounding tissue and the pKa are equal, 50% of each ionic form will exist. As the pKa of the local anesthetic increases or as the pH of the surrounding environment is reduced, a greater proportion of the charged, relatively impermeable cationic species will exist (see Table 3-2 ).
When the local anesthesia is injected into an inflamed or acidic environment, the more hydrophilic portion of the drug will be preponderant, resulting in decreased neuronal penetration and potency. At physiologic pH of 7.4 or higher, local anesthetics with a lower pKa, such as mepivacaine, lidocaine, and prilocaine, will have a higher percentage of their free base available for neuronal diffusion. For instance if lidocaine with a pKa of 7.8 is injected into a site with a pH of 6.0, the proportion of the cationic form will increase from 74% to 98%. This leaves only 2% of the local anesthetic capable of penetrating the nerve sheaths, making complete anesthesia improbable. Other studies show that in addition to pH there are other local mediators of inflammation, such as prostaglandins and bradykinin, that can antagonize the effects of local anesthetics. Local anesthetics are typically manufactured as an acidic hydrochloride salt, with a pH ranging from 3.5 to 6.0. This improves the water solubility of the local anesthetic and stabilizes the vasoconstrictor. Because of the low pH, approximately 99% of local anesthetic will carry a positive charge. As a result of the buffering capacity of the soft tissue, it is only after injection that local anesthetic is converted into the free base form, capable of penetrating the nerve sheaths.
▪
MECHANISM OF ACTION OF LOCAL ANESTHETICS
Local anesthetics can be used in three modes: topical application, local infiltration, and nerve blockade. All will lead to the blockage of the sensation of pain, touch, temperature, and proprioception by interfering with the propagation of impulses along peripheral nerve fibers. This is accomplished by reduction in the rate rise in the depolarization phase, leading to a slowing of the action potential. At the molecular level, this deterioration in the action potential is accomplished by blocking the influx of sodium through the excitable nerve membranes. By completely abolishing the inward movement of depolarizing sodium while having little effect on outward movement of repolarizing potassium, the action potential is blocked. Additionally, based on experiments on isolated nerve preparations, there is a direct relationship between the concentration of sodium in surrounding tissue and concentration of local anesthetic needed to completely block the action potential. As the gradient for influx of sodium becomes more favorable, more local anesthetic is needed to block the action potential.
At sodium channels, both the basic and cationic species appear to be active, with the cationic species entering the sodium channels when they are in the open state and the free base form entering the sodium channels when they are open or closed. In a classic study carried out by Ritchie et al, nerves still possessing their outer covering were most susceptible to local anesthetics at alkaline pH. Yet when the epineurium was stripped off the nerve fibers, the local anesthetics were most effective at the more acidic pH. These experiments confirmed the notion that the cationic form of the local anesthetics was also effective in blocking the propagation of action potentials. Thus the sequence of events induced by local anesthetic molecules is as follows: (1) a reduction in the permeability of the nerve cell membrane to sodium ions, (2) a decreased rate of rise in the depolarization phase of the action potential, and (3) failure of a propagated action potential.
▪
POTENCY OF LOCAL ANESTHETICS
Potency is a comparative term that estimates the amount of one drug relative to another, in terms of how much of each drug is needed to have same effect. Potency and duration of action can differ between each drug depending on the chemical structure and the presence of other additives, such as vasoconstrictors, which will be discussed later. Generally speaking the more hydrophobic or lipophilic the agent, the greater the potency and the longer the duration of action. As shown in Table 3-3 , potency is directly related to lipid solubility and inversely related to the concentration of the local anesthetic. The lower the lipid solubility, the higher the concentration of local anesthetic that is needed to produce the same effect. For this reason, local anesthetics with higher lipid solubility are manufactured at lower concentrations.
| Drug | Lipid Solubility | Concentration |
|---|---|---|
| Articaine | 40 | 4% |
| Mepivacaine | 42 | 2%-4% |
| Prilocaine | 55 | 4% |
| Lidocaine | 110 | 2% |
| Bupivacaine | 560 | 0.5% |
The lipid solubility of local anesthetics can be manipulated by the addition or removal of carbon atoms at key sites on the molecule. For example, as shown in Figure 3-2 , the addition of a butyl group (-C 4 H 9 ) to mepivacaine results in the formation of bupivacaine, which possesses at least four times the relative potency from a clinical standpoint. Mepivacaine is marketed at a 2% to 4% solution, whereas bupivacaine is marketed as a 0.5% solution. However, when comparing the two from a potency standpoint, there is no evidence that one local anesthetic is superior to another in the laboratory or in the clinical setting.
▪
EFFICACY AND DURATION OF ACTION OF LOCAL ANESTHESIA
There are various factors that influence the efficacy and the duration of local anesthetic action. First, and the most obvious, is accurate anatomic placement of the local anesthetic in the vicinity of the desired nerve. This is especially true of the mandibular nerve, which has been shown to possess many accessory branches. The second factor has to do with the actual chemical makeup of the local anesthetic. Properties, such as the solubility of the local anesthetic into the nerve sheath or the presence or absence of a vasoconstrictor, along with the intrinsic activity of the local anesthetic, can make a difference in the efficacy and duration of action. As a general rule, the duration of anesthesia in soft tissues lasts longer than pulpal anesthesia by a factor of 2 to 3. This is shown in Table 3-4 and holds true even in the presence or absence of vasoconstrictors. For the listed local anesthetics, there is a range of maximum dosage recommendations, with the number in parenthesis based on Malamed’s more conservative guidelines. The higher numbers are based on the package inserts and Jastak et el.
| Anesthetic | Duration in Minutes | |||||||
|---|---|---|---|---|---|---|---|---|
| Maxillary Infiltration | Mandibular Block | Maximum Dosage | Maximum Total | |||||
| Pulp | Soft Tissue | Pulp | Soft Tissue | mg/kg | mg/lb | Dosage (mg) | ||
| Lidocaine | 2% plain | 5 | 60 | 10 | 120 | 4.5 | 2.0 | 300 |
| 2% + 1 : 50,000 epinephrine | 60 | 180 | 90 | 240 | 7.0 (4.5) | 3.18 (2.0) | 500 (200) * | |
| 2% +1 : 100,000 epinephrine | 60 | 180 | 85 | 240 | 7.0 (4.5) | 3.18 (2.0) | 500 (300) | |
| Mepivacaine | 3% plain | 20 | 120 | 40 | 180 | 6.6 (4.5) | 2.2 (2.0) | 400 (300) |
| 2% + 1 : 100,000 epinephrine | 60 | 170 | 85 | 190 | 6.6 (4.5) | 2.2 (2.0) | 400 (300) | |
| 2% + 1 : 20,000 levonordefrin | 45 | 120 | 75 | 240 | 6.6 (4.5) | 2.2 (2.0) | 400 (300) | |
| Articaine | 4% + 1 : 100,000 epinephrine | 60 | 180 | 75 | 360 | 7.0 [5.0] † | 3.2 [2.72] † | 500 [144] † |
| 4% + 1 : 200,000 epinephrine | 45 | 120 | 60 | 300 | 7.0 [5.0] † | 3.2 [2.72] † | 500 [144] † | |
| Prilocaine | 4% plain | 20 | 105 | 60 | 240 | 8.0 (6.0) | 3.64 (2.7) | 600 (400) |
| 4% + 1 : 200,000 epinephrine | 45 | 120 | 90 | 240 | 8.0 (6.0) | 3.64 (2.7) | 600 (400) | |
| Bupivacaine | 0.5% +1 : 200,000 epinephrine | 45 | 240 | 240 | 540 | 1.3 | 0.6 | 90 |
* Based on the 200 mg of epinephrine limit.
† Represents maximum recommended dose in children ≤30 kg or ≤13.6 lb.
Long-acting local anesthetics, such as bupivacaine, are highly lipid soluble and bind more tightly to protein and lipid structures within the nerve membrane than other local anesthetics. When administered via mandibular block, these long-acting anesthetics with 1 : 200,000 epinephrine possess a longer duration of pulpal anesthesia than other marketed anesthetic solutions. However, several studies of infiltrative or PDL injections of bupivacaine with 1 : 200,000 epinephrine show that in the maxilla the pulpal anesthesia duration is not longer, and in some cases even shorter, than the duration of 2% lidocaine with 1 : 100,000 epinephrine.
It is important to note that local anesthetics have an intrinsic vasodilatory property. This is especially true of lidocaine, which in the absence of a vasoconstrictor, is rapidly redistributed away from the site of injection. As shown in Table 3-4 , 2% lidocaine plain without epinephrine maintains pulpal anesthesia duration of about 5 minutes in the maxilla and 10 minutes in the mandible. Adding 1 : 100,000 epinephrine to this solution increases the duration of pulpal anesthesia approximately tenfold, to 60 minutes in the maxilla and 85 minutes in the mandible. In contrast both mepivacaine and prilocaine have less vasodilatory activity than lidocaine. Solutions of 3% mepivacaine or 4% prilocaine in the absence of a vasoconstrictor can produce pulpal anesthetic durations in the 20- to 55-minute range, which is only doubled in the presence of a vasoconstrictor.
▪
USE OF VASOCONSTRICTORS WITH LOCAL ANESTHETICS
For decades the use of vasoconstrictors with local anesthesia has been one of the most contentious topics in dentistry. Considering today’s aging population and their comorbidities, this issue will surely continue to be contentious. Because of the potential adverse effects of vasoconstrictors, it has been unethical to perform controlled, randomized, and double-blind studies on humans. Much of what has been debated is based on anecdotal observations on human case reports and animal studies with unsubstantiated data. Questions still remain as to the actual interplay of vasoconstrictors and cardiovascular disease and the dreaded drug-drug interactions.
Today the two most common vasoconstrictors used in dental anesthetic solutions in the United States are epinephrine and levonordefrin. Local anesthetics containing NE are no longer manufactured in the United States, but continue to be used elsewhere in the world. In general vasoconstrictors are classified as adrenergic or sympathomimetic because they are, or closely resemble, the natural mediators of the sympathetic nervous system. Chemically, they are classified as catecholamines because of their direct action on the adrenergic receptors. By design vasoconstrictor concentrations within local anesthetics are titrated to approximate the same α-adrenergic activity. This explains the varying concentration of vasoconstrictors that are found in commercial formulations.
The adrenergic system is composed of both β and α receptors. Each receptor type is further subdivided into β 1 or β 2 and α 1 or α 2 . For the most part, the β-adrenergic system is mostly systemic, whereas the α-adrenergic system is mainly peripheral with less systemic action. The main action of the β 1 receptor is at the heart, where its activation increases heart rate and automaticity of the heart via the SA node, along with increasing contractility and conduction of the heart. β 2 receptors are located in the vascular beds of skeletal muscles and pulmonary vasculature. Their activation leads to vasodilation or relaxation of the trachea and bronchioles. On the other hand, α 1 receptors are found in the peripheral vasculature and cause severe vasoconstriction of the peripheral arterioles and veins on stimulation. For this reason, the use of local anesthetic with vasoconstrictors is contraindicated in distal appendages with an end vascular supply, such as nose, ears, fingers, and toes. Lastly the stimulation of α 2 receptors, which are mostly found in the CNS, leads to decreased sympathetic outflow from the brain and a decrease in the release of NE from the presynaptic neurons.
The physiologic result of β 1 stimulation is an increase in blood pressure, whereas the stimulation of β 2 receptors tends to decrease blood pressure. The α receptors mainly cause vasoconstriction at the peripheral circulation, skin, and mucous membranes, with a nominal increase in the blood pressure. For instance the antihypertensive medication prazosin (Minipress), which is an α 1 antagonist, has only limited effectiveness against minimal to moderate hypertension with negligible decreases in blood pressure of approximately 10 to 12 mm Hg. Fortunately, because of their similarity to endogenous catecholamines, their action is terminated within one minute by the enzyme catechol-O-methyl transferase (COMT), which is readily available in blood, liver, lungs, and other tissues.
Examining the actions of catecholamines’ subtle differences are noted. Table 3-5 shows that epinephrine is the most potent with respect to α receptor stimulation, as compared with NE, levonordefrin, and phenylephrine. NE stimulates the β 1 receptor preferentially over β 2 , therefore causing an increase in blood pressure when used as a vasoconstrictor in local anesthetic. On the other hand, epinephrine has an equal affinity for both α and β receptors, causing no dramatic increases in blood pressure as a result of β 2 vasodilation. The vasodilatory β 2 receptors are believed to be more sensitive to low blood levels of epinephrine than are the vasoconstrictive α 1 receptors. This explains why small doses of epinephrine often increase heart rate and systolic blood pressure yet actually reduce diastolic blood pressure, with the mean arterial pressure (MAP) remaining unchanged.
| Vasoconstrictor | β 1 Selectivity | β 2 Selectivity | Relative α Potency |
|---|---|---|---|
| Epinephrine | 50% | 50% | 100% |
| Norepinephrine | 85% | 15% | 25% |
| Levonordefrin | 75% | 25% | 15% |
| Phenylephrine | 95% | 5% | 5% |
Because of NE’s mostly β 1 properties, local anesthetics containing NE have resulted in numerous adverse reactions during routine dental treatment. Some of these reactions were attributed to “rebound bradycardia,” which is a compensatory response to the initial hypertension caused by β 1 stimulation. Additionally, NE has shown to impair left ventricular diastolic function, which can be detrimental in patients with congestive heart failure. As a result of systemic side effect, NE was removed from most local anesthetic formulations in the United States.
Levonordefrin, which is the least potent of the catecholamines, is most similar to NE, possessing less α 1 but slightly more β 2 action. It is still available in a 1 : 20,000 solution (0.05 mg/mL). At low blood levels, it closely resembles NE’s pressor effects. Even though NE and levonordefrin have less α 1 activity, one may think that they would be preferred over epinephrine in patients with heart disease. On the contrary, with the preservation of the patient’s ability to increase their peripheral vascular resistance more stress can be placed on the heart when NE or levonordefrin is given. Hence patients with documented hypertension should not receive NE or levonordefrin because both will exert unrestricted activation of α 1 receptors, which could lead to uncontrolled increases in blood pressure. This is especially true in patients with severe, uncontrolled hypertension; refractory arrhythmia, myocardial infarction, or stroke within 6 months; uncontrolled congestive heart failure; and uncontrolled hyperthyroidism.
POTENTIAL BENEFITS OF VASOCONSTRICTORS
The addition of vasoconstrictors to local anesthetics arose from the desire to reduce or prevent the redistribution of the local anesthetics away from the site of injection. As an obvious strategy to reduce the vascular removal of the local anesthetic, vasoconstrictors, such as epinephrine, were the natural choice. It is now known that the use of vasoconstrictors decreases the clearance of the local anesthetic, reduced the total required amount, increases the duration and depth of anesthesia, and aids in hemostasis of the surgical wound. Studies show that with the presence of a vasoconstrictor there is a delay and a reduction in the peak blood levels of the local anesthetic. *
* .
This is of importance because excessive blood levels of local anesthetics are known to cause systemic toxicity, especially in the pediatric dental population.
Additionally, local anesthetics, especially lidocaine, inherently cause vasodilation, further strengthening the need for vasoconstrictors. Experiments show that with the addition of vasoconstrictors the duration of action of all local anesthetics is prolonged. This is especially true with lidocaine instead of mepivacaine or prilocaine. As shown in Table 3-4 , 2% plain lidocaine possesses a pulpal anesthesia of about 5 to 10 minutes. With the addition of 1 : 100,000 epinephrine, the duration is increased by ten-fold to approximately 60 minutes. This increase in the duration of action is less dramatic with bupivacaine, possibly as a result of its higher lipophilic properties. In one study with the absence of a vasoconstrictor, 2% plain lidocaine did not predictably produce pulpal anesthesia, and the efficacy of anesthesia was only 38%, obviously unacceptable in a clinical setting.
Some of the inherent properties of vasoconstrictors with local anesthetics were demonstrated in a recent study by Sinnott in 2000, where rat sciatic nerves were blocked using 0.5% lidocaine with or without epinephrine. As expected the use of epinephrine greatly potentiated the duration of action by four-fold. They were able to show that only after 5 minutes the total intraneural lidocaine doubled with the presence of epinephrine, and the rate at which it declined was insignificantly decreased by the vasoconstrictor. This trend was attributed to epinephrine’s action early in the blockade. By enhancing the initial neural uptake of the local anesthetic and sustaining a higher concentration of local anesthetic in the surrounding tissue, a higher concentration gradient was created, driving the local anesthetic deeper into the core of the nerve. This results in less local anesthetic needed to achieve the desired nerve block.
The presence of vasoconstrictor also seems to be beneficial in patients with cardiovascular disease because it reduces the release of reactionary endogenous NE. Dental anxiety, fear, anesthesia, and surgery are all accompanied by physiologic, psychological, and biochemical changes. Physiologically, this translates into changes in the heart rate and blood pressure, cardiac arrhythmias, and release of endogenous catecholamines. Multiple studies show that in dental treatment, even with adequate anesthesia, there are increased levels of endogenous catecholamines. In turn significant increases in blood pressure can be seen, leading to adverse reactions. In a randomized, blinded, controlled, and comparative study, it was noted that perioperative anxiolytics, such as midazolam (Versed), may attenuate the sympathoadrenal response. This was further supported by a Swedish study, where a randomized controlled trial of 35 atypical odontalgia patients in Sweden reported that lidocaine injections provided significant, but not complete, pain relief as compared with saline (placebo) injections. This suggests the pain experienced in these patients is not solely dependent on peripheral afferent inputs and must involve higher order neurons. When compared with 0.15 mg/kg of midazolam (Versed), 1.5 μg/kg of clonidine (Catapres) and 0.3 mg/kg morphine were found to be the more effective premedications in reducing pain during application of local anesthesia. This suggests that clonidine (Catapres) should be selected over morphine as a premedication to reduce anxiety and pain.
Today it is generally agreed that the concentration of vasoconstrictors needs to be somewhere between 1 : 200,000 and 1 : 100,000 to improve the duration of action. Increasing the concentration of any vasoconstrictor to 1 : 50,000 does not only cause significant cardiovascular changes but has also not shown to be of any benefit in duration of action. In a study of 11 normotensive patients undergoing tooth extraction with 2% lidocaine with epinephrine (1 : 80,000), it was found that during surgery there was a significant increase in systolic blood pressure. Furthermore the serum glucose levels increased after local anesthesia; plasma insulin concentrations remained steady. Together these data suggest sympathoadrenal outflow is activated by administration of local anesthetic and tooth extraction, which ultimately leads to an increase in serum glucose levels in patients with normal blood pressure. This is in addition to the stress of the procedure.
As mentioned previously, another advantage of using vasoconstrictors in local anesthetic is the hemostatic effect it provides at the surgical site. In one third molar study, there was a 50% reduction in blood loss with the use of 2% lidocaine plus 1 : 100,000 epinephrine versus 3% plain mepivacaine. Hemostasis also lends itself to a more visible surgical field and decrease in the risk of aspiration of blood, especially during intraoral procedure. It is important to note that the optimum concentration of local anesthetic that is needed for adequate hemostasis is specific to each local anesthetic. Studies clearly indicated that with lidocaine a concentration of 1 : 50,000 epinephrine is more effective than the less concentrated solutions of 1 : 200,000. Along the same lines, 1 : 200,000 mixture of epinephrine seems to impart adequate hemostasis when used with prilocaine and bupivacaine.
POTENTIAL SYSTEMIC SIDE EFFECTS OF VASOCONSTRICTORS
Even though vasoconstrictors reduce systemic absorption of the local anesthetic, there is still systemic absorption of the vasoconstrictor itself, especially if there is an inadvertent intravascular injection. Fortunately, even with systemic absorption of vasoconstrictors, the blood pressure and heart rate remains unchanged, with minimal effect on the MAP. This is despite the fact that investigators repeatedly report a two-fold to four-fold increase in the basal epinephrine levels after an injection of local anesthetics with 1 : 100,000 epinephrine. It is important to note that plain lidocaine had no effect on plasma epinephrine levels or any cardiovascular parameter.
Because the majority of patients undergoing elective extraction of teeth are young and healthy adults, their cardiovascular response is expected to be different than a 50-year-old patient with significant atherosclerotic and/or cardiovascular disease. In a typical third molar extraction case, it is not unusual to administer eight cartridges of 2% lidocaine plus 1 : 100,000 epinephrine. That equates to 288 mg of local anesthetic plus 144 μg of epinephrine. In one particular study, this amounted to a twenty-seven-fold increase in systemic epinephrine levels, resulting in an approximate elevation in systolic blood pressure of about 20 mm Hg, an increase in the heart rate of about 20 beats per minute, and a 52% increase in myocardial oxygen consumption. This may explain why most fatal outcomes have been in older patients with significant cardiovascular disease.
So it seems that even with the use of local anesthetics the likelihood of systemic toxicity cannot be completely eliminated. This is especially true of inadvertent intravascular administration of epinephrine, which has been shown to redirect a larger than expected percentage of cardiac output toward the brain, doubling the delivery of lidocaine to an organ that is most susceptible during local anesthetic overdoses. In the event that vasoconstrictors cannot be used, 2% to 3% mepivacaine or 4% prilocaine are the two dental local anesthetics that can provide acceptable pulpal anesthesia without the use of vasoconstrictors.
POTENTIAL DRUG-DRUG INTERACTION WITH VASOCONSTRICTORS
Drug-drug interaction with vasoconstrictors is one area that continues to evolve. Over the years, the risk of drug interactions with vasoconstrictors has been overstated in some patient groups, and it remains underappreciated in others. There are two articles that are repeatedly cited as cause for concern when dealing with drug-drug interactions as related to antidepressant or antihypertensive medications. Yet an increasing body of evidence is beginning to question some of what were once thought to be absolute drug-drug interactions. Before any conclusions can be drawn, more well-designed studies are needed.
Table 3-6 summarizes the three main categories of antidepressant medications: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), and selective serotonin reuptake inhibitors (SSRIs). In the past, it was widely believed that the use of MAOIs and TCAs, excluding SSRIs, were an absolute contraindication to the use of vasoconstrictors. However, in the past 30 years, there has not been a single case report of an adverse reaction with local anesthetics plus vasoconstrictors in the millions of patients taking these antidepressant medications. The interaction of MAOIs with local anesthetics plus vasoconstrictors was thought to be related to the accumulation of serum catecholamines. In turn this was postulated to cause an increased risk of severe hypertension or cardiac arrhythmia. However, the short half-life of exogenously administered catecholamines and their quick reuptake in the nerve terminals makes it unlikely that such an accumulation can take place. Furthermore in animal studies, the concurrent administration of any catecholamines with the MAOI phenelzine did not produce any cardiovascular findings. A potential drug interaction with MAOIs should only be a concern with certain adrenergic drugs, such as phenylephrine and ephedrine, which have a noncatecholamine structure.
| Antidepressant Classification | Generic Name | Trade Name |
|---|---|---|
| Monoamine oxidase inhibitors (MAOIs) | Isocarboxazid | Marplan |
| Phenelzine | Nardil | |
| Selegiline transcutaneous | Emsam | |
| Tranylcypromine | Parnate | |
| Tricyclic antidepressant (TCAs) | Amitriptyline | Elavil |
| Clomipramine | Anafranil | |
| Desipramine | Norpramin | |
| Doxepin | Sinequan | |
| Imipramine | Tofranil | |
| Nortriptyline | Aventyl HCL, Pamelor | |
| Protriptyline | Vivactil | |
| Trimipramine | Surmontil | |
| Selective serotonin reuptake inhibitors (SSRIs) | Citalopram | Celexa |
| Escitalopram | Lexapro | |
| Fluoxetine | Prozac, Sarafem | |
| Fluvoxamine | Fluvox | |
| Paroxetine | Paxil, Pexeva | |
| Sertraline | Zoloft |
With regard to TCAs, there have been three commonly cited studies speaking of a potentially dangerous increase in blood pressure in patients taking TCAs. However, none of these studies were blinded or randomized, and their statistical power was low. Interestingly, in one of these studies by Yagiela et al, even though no significant change was noted in the MAP for the dogs tested with epinephrine, dogs pretreated with desipramine had significant increases in MAP when NE or levonordefrin were used. Nevertheless, without distinguishing between NE and epinephrine, the interaction between vasoconstrictors and TCAs was deemed serious enough to deserve special attention. These studies also failed to take into account the receptor dynamics. Antidepressant medications, like other chemicals, are known to cause down-regulation of CNS β-adrenergic receptors, only 2 to 3 weeks after the initiation of drug therapy. Because the subjects in the above studies were given TCAs for periods of 5 days or less, the down-regulation of the β-adrenergic receptors never took place. This may cause one to think that the so-called hypertensive reactions took place in TCA-naive subjects rather than in adrenergic receptor down-regulated subjects.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


