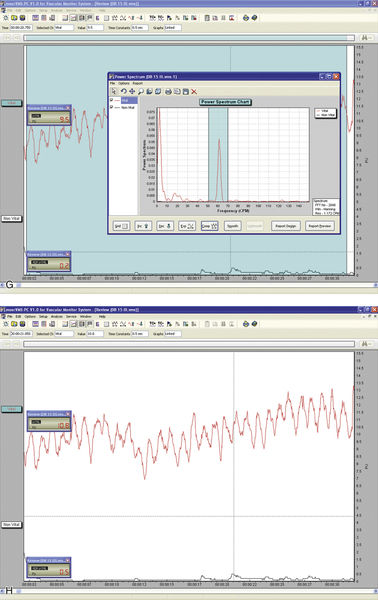
• Figure 13-1 A, Laser Doppler flowmeter unit. B, Mold made of quick-set putty with two embedded laser Doppler probes. C, Slow-speed round burs can be used to make holes in the putty for the placement of probes. D and E, Laser Doppler probes for assessment of blood flow in anterior teeth (D) and posterior teeth (E). F, Quick-set putty with dental probes in position in the mouth. G, Blood flow analysis readout. H, Fourier analysis of blood flow data. (A, D, E, G, H, Courtesy Moor Instruments, Ltd.)
Moritz et al.29 used a CO2 laser in patients requiring direct pulp-capping treatment. Laser irradiation at an energy level of 1 W with 0.1-second exposure time and a 1-second pulse interval was repeated until the exposed pulp cavities were completely sealed. The pulps were then dressed with Ca(OH)2. In the control group, the pulps were capped with Ca(OH)2 only. Symptom status and tooth vitality were assessed after 1 week and monthly for 1 year; 89% of patients in the experimental group had no symptoms and exhibited normal responses to vitality tests in the treated pulps versus only 68% in the control group.
With deep and hypersensitive cavities, indirect pulp capping should be considered. A reduction in the permeability of the dentin, achieved by sealing the dentinal tubules, is paramount. Nd:YAG and 9.6-μm CO2 lasers can be used for this purpose. The 9.6-μm CO2 laser wavelength is well absorbed by the hydroxyapatite of enamel and dentin, causing tissue ablation, melting, and resolidification.30 The use of the 9.6-μm CO2 laser did not cause any noticeable damage to the pulpal tissue in dogs.31
White et al.32 found that use of a pulsed Nd:YAG laser with an energy level less than 1 W, with a 10-Hz repetition rate and overall 10-second exposure time, did not significantly elevate the intrapulpal temperature. According to their results, these settings may be considered safe parameters because the remaining dentinal thickness in cavity preparations cannot be measured in vivo. It is therefore recommended that clinicians choose laser parameters lower than these safety limits.
Cleaning and Disinfecting the Root Canal System
Bacterial contamination of the root canal system is considered the principal etiological factor in the development of pulpal and periapical lesions.33–35 Creating a root canal system free of irritants is a major goal of root canal therapy, traditionally achieved through biomechanical instrumentation. Because of the complexity of the root canal system, however, complete elimination of debris resulting in a sterile root canal system is difficult.36,37 Also, a smear layer, which covers the instrumented walls of the root canal, is formed during this treatment.38–40
The smear layer consists of two parts: a superficial layer on the surface of the root canal wall approximately 1 to 2 μm thick and a deeper layer packed into the dentinal tubules to a depth of up to 40 μm.40 It contains inorganic and organic substances that include microorganisms and necrotic debris.41 In addition to possible infection of the smear layer itself, it also can protect the bacteria already present deeper in the dentinal tubules by preventing intracanal disinfection agents from penetrating into the tubules.42 Pashley43 also has postulated that a smear layer containing bacteria or bacterial products might provide a reservoir of irritants. Thus, complete removal of the smear layer would be consistent with elimination of irritants from the root canal system.44
In addition, Peters et al.45 clearly demonstrated that more than 35% of the canals’ surface area remained unchanged after instrumentation of the root canal using four different nickel-titanium (NiTi) preparation techniques. Because most current intracanal medicaments have a limited antibacterial spectrum and limited ability to diffuse into the dentinal tubules, development of newer treatment strategies designed to eliminate microorganisms from the root canal system should be considered. Such strategies must include use of agents that can penetrate the dentinal tubules and destroy the microorganisms located in an area beyond the host defense mechanisms, where they cannot be reached by systemically administered antibacterial agents.46
Numerous studies also have documented that CO2,47 Nd:YAG,47–49 argon,47,50 Er,Cr:YSGG,51 and Er:YAG52,53 laser irradiation have the ability to remove debris and the smear layer from the root canal walls after biomechanical instrumentation.
However, the intracanal use of lasers has several limitations.54 The emission of laser energy from the tip of the optical fiber or the laser tip is directed along the root canal and not necessarily laterally along the root canal walls.55 Thus, it is almost impossible to obtain uniform 360-degree coverage of the internal aspect of the root canal system surface using a laser.54,55 An important additional consideration, in view of the risk of thermal damage to the periapical tissues, is safety.55 Direct emission of laser radiation from the tip of the optical fiber in the vicinity of the apical foramen of a tooth may result in transmission of the energy beyond the foramen. This in turn may adversely affect the supporting tissues of the tooth, which can be hazardous in teeth close to the mental foramen or to the mandibular nerve.55,56
Matsumoto3 emphasized the possible limitations of laser use in root canal systems, suggesting that “removal of smear layer and debris by laser is possible, however it is difficult to clean all root canal walls, because the laser is emitted straight ahead, making it almost impossible to irradiate the lateral canal walls.” These workers researchers strongly recommended improving laser endodontic tips to enable irradiation of all areas of the root canal walls.
Erbium lasers have gained increasing popularity among clinicians after their clearance by the U.S. Food and Drug Administration (FDA) for use on hard dental tissues.57 Stabholz et al.55,56 described a newer endodontic tip that can be used with an erbium laser system. The beam of the erbium laser is delivered through a hollow tube, permitting development of an endodontic tip that allows lateral emission of the laser radiation (side-firing) rather than direct emission through a single opening at the far end. This new endodontic side-firing spiral tip was designed to fit the shape and the volume of root canals prepared by NiTi rotary instrumentation. It emits the erbium laser radiation laterally to the walls of the root canal through spiral slits located all along the length of the tip. The tip is sealed at its far end, preventing the transmission of radiation to or through the apical foramen of the tooth. In an evaluation of the efficacy of the endodontic side-firing spiral tip in removing debris and smear layer from distal and palatal root canals of freshly extracted human molars, scanning electron microscopy (SEM) of the treated root canal walls revealed clean surfaces, free of smear layer and debris (Figures 13-2 to 13-5).56
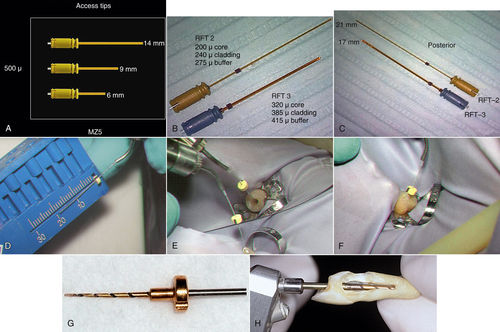
• Figure 13-2 A to C, Er,Cr:YSGG laser tips can be used to create endodontic access openings (A) and for instrumentation of canals in anterior teeth (B) and posterior teeth (C). D to F, Er:YAG laser tip being fitted for a stopper for working length minus 1 mm for endodontic canal (D), entering the canal (E), and reaching measurement depth (F). G, RCLase Side-Firing Spiral Tip (Opus Dent, Tel Aviv, Israel). H, Prototype of RCLase Side-Firing Spiral Tip is shown in the root canal of an extracted maxillary canine in which the side wall of the root has been removed to enable visualization of the tip. (A to C courtesy Dr. David Browdy, Lynbrook, New York; D to F courtesy Dr. Donald Coluzzi, Redwood City, California.)
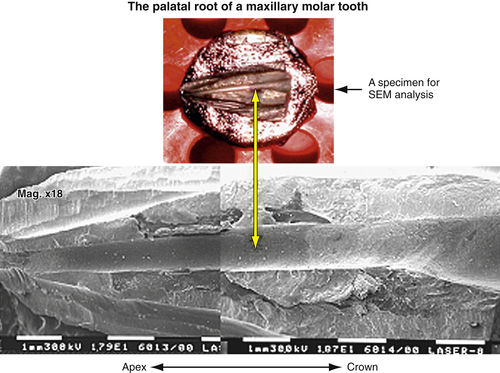
• Figure 13-3 Longitudinally split palatal root of maxillary molar, sputter-coated by gold and ready for scanning electron microscope (SEM) evaluation. Vertical arrow indicates the root canal as shown on SEM photograph.

• Figure 13-4 A to C, Scanning electron microscope photographs of laser-treated wall of root canal at its apical (A), middle (B), and coronal (C) portions demonstrate clean surfaces of root canal walls, free of smear layer and debris, and clean, open dentinal tubules. (Magnification ×300.)
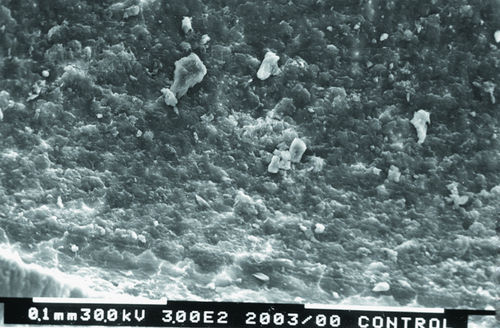
• Figure 13-5 Scanning electron microscope photograph of a non-laser-treated wall of root canal at its middle portion demonstrates unclean surfaces of root canal walls, with smear layer and debris. Dentinal tubules cannot be seen. (Magnification ×300.)
The dentinal tubules in the root run a relatively straight course between the pulp and the periphery, in contrast to the typical S-shaped contours of the tubules in the tooth crown.41 Studies have shown that bacteria and their byproducts, present in infected root canals, may invade the dentinal tubules. Also, the presence of bacteria in the dentinal tubules of infected teeth was noted at approximately one half the distance between the root canal walls and the cementodentinal junction.58,59 These findings justify the rationale and need for developing effective means of removing the smear layer from root canal walls after biomechanical instrumentation. This cleansing step would allow disinfectants and laser energies to reach and destroy microorganisms within the dentinal tubules.
In various laser systems used in dentistry, the emitted energy can be delivered into the root canal system by a thin optical fiber—as in Nd:YAG, potassium titanyl phosphate (KTP)–Nd:YAG, Er:YSGG, argon, and diode lasers—or by a hollow tube—as in CO2 and Er:YAG lasers. Thus, the potential bactericidal effect of laser irradiation can be effectively used for additional cleansing and disinfecting of the root canal system after biomechanical instrumentation. This effect was extensively studied using CO2,60,61 Nd:YAG,62–65 KTP-Nd:YAG,66 excimer,67,68 diode,69 and Er:YAG70–72 lasers.
The apparent consensus is that laser irradiation performed using dental laser systems has the potential to kill microorganisms. In most cases the effect is directly related to the amount of irradiation and to its energy level. Case Study 13-1, with Figure 13-6, illustrates the use of erbium laser energy to clean and disinfect a root canal system.
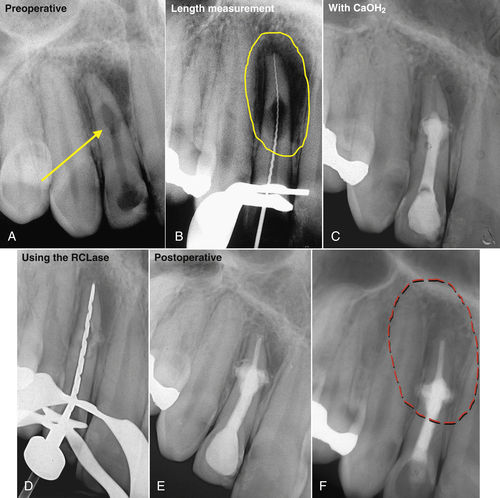
• Figure 13-6 Laser-assisted root canal filling. A, Preoperative radiographs of the maxillary right incisor showing internal resorption. B, A root perforation is visible on the measurement radiograph. C, Tooth filled with calcium hydroxide. D, Laser tip in canal. E, Maxillary lateral incisor after completion of root canal filling using a hot gutta-percha technique. F, Two-year follow-up radiograph reveals complete healing of periapical lesion. The sinus tract has disappeared, and the patient is free of symptoms.
DeVito et al. have described a new method of removing the smear layer and disinfecting root canals using an Er:YAG laser: These researchers have patented a procedure called Photon Induced Photoacoustic Streaming (PIPS). According to their work, at subablative parameters, when an Er:YAG laser is activated in a root canal, the strong absorption of the wavelength in water creates a shockwave, which removes the smear layer and disinfects the canals. An in vitro study of 80 single-rooted extracted human teeth demonstrated pronounced smear layer removal with no thermal damage to the dentinal walls, and thermocouple studies have shown that the root surface temperature increases were well within an acceptable level.73
Lloyd et al. used 14 extracted human mandibular molars to compare single-needle irrigation of the root canal systems with photon-induced photoacoustic streaming. They concluded that the laser technique eliminated debris better than single-needle irrigation.74 Peters et al. studied the efficacy of canal disinfection using sodium hypochlorite irrigation, sodium hypochlorite ultrasonically activated; and Er:YAG laser. These researchers concluded that all three techniques disinfected the root canal systems, with the laser treatment the most effective modality.75 Other in vitro studies76,77 have replicated the results of the various investigations. These in vitro studies have established proof of principle for this technique; however, longitudinal in vivo studies evaluating the long-term success rate of teeth treated endodontically with this new technique versus more conventional techniques need to be published to validate this treatment.
Obturation of the Root Canal System
Obturation of the prepared root canal space is done (1) to eliminate all avenues of leakage from the oral cavity or from the periradicular tissues into the root canal system and (2) to seal within the system any irritants that cannot be fully removed during the cleaning and shaping procedures.78 The rationale of introducing laser technology to assist in obturating the root canal system is based on the following two assumptions about the laser’s ability:
• Using the laser irradiation as a heat source for softening gutta-percha, which is employed as the obturating material
• Using the laser as a means to condition the dentinal walls before placement of an obturation bonding material
The concept of thermoplasticized compaction is not new and covers any technique that is based entirely on the heat softening of gutta-percha combined primarily with vertical compaction. The technique has many different names, including warm sectional technique, vertical compaction with warm gutta-percha, and the Schilder technique. Schilder79 first described the technique to fill root canals in three dimensions more than 40 years ago. At present, some dental practitioners still use this technique, whereas others use newer, warm gutta-percha techniques such as thermomechanical compaction, thermoplasticized gutta-percha, and injection of softened gutta-percha, which have been introduced to simplify the root canal filling procedure.
The first laser-assisted root canal filling procedure used the wavelength of the 488-nm argon laser. This wavelength, which is transmitted through dentin, was used to polymerize a resin placed in the main root canal. Testing the ability of this biomaterial to penetrate into accessory root canals showed that the resin in the lateral canals was readily polymerized at low energy levels (30 mW). Further use of this wavelength became irrelevant because of its poor properties for most other dental procedures.80
Anic and Matsumoto81 were the first to compare different root canal filling techniques used to fill single-rooted teeth. Lateral condensation, vertical condensation, low-temperature gutta-percha (Ultrafil), and laser-cured resin with different wavelengths (argon, CO2, Nd:YAG) were used. The apical sealing ability achieved by the various filling techniques was compared by measuring apical dye penetration after placement of the samples in a 1% solution of methylene blue. Gutta-percha softened with an argon laser created an apical seal similar to that obtained with the lateral condensation and Ultrafil techniques.
Maden et al.82 used the dye penetration method to measure apical leakage by comparing lateral condensation, System B technique (using thermoplasticized compaction; SybronEndo, Orange, California), and Nd:YAG-softened gutta-percha. No statistically significant differences among the different treatment groups were reported. Anic and Matsumoto83 measured a temperature elevation on the outer root surface of 14.4° C with use of the Nd:YAG and of 12.9° C with the argon laser. Such a temperature increase may be detrimental to the tissues of the periodontal ligament and attachment apparatus of the teeth, so the implications of using such treatment methodologies remain in question.
To examine whether laser irradiation does improve the adhesion of endodontic materials to the dentinal walls of the root canal and reduce apical leakage, Park et al.84 used different sealers and two root canal filling techniques. These investigators concluded that Nd:YAG laser irradiation at the end of the root canal preparation (at 5 W, 20 Hz) reduced the apical leakage regardless of the sealer or the technique used. Kimura et al.85
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses