The concept of osseointegration has revolutionized the treatment options for the replacement of missing teeth in both partially and completely edentulous patients. Dental implants are widely used because clinical practice and studies have documented its successful outcomes. However, implants can occasionally fail, and such failures can be classified as early or late. Measures that can aid in the early recognition of failing osseointegrated implants are needed, as are measures that can facilitate appropriate treatment methods aimed at saving failing implants by determining the probable etiologic factors. This article summarizes our current understanding of the local factors that can be linked to implant failure.
Key points
- •
Clinicians should critically evaluate the patient’s oral hygiene, compliance, motivation, and risk factors before suggesting dental implant treatment.
- •
Achieving primary stability is important for successful implant placement.
- •
Host-related factors, operative-related factors, and implant-related factors may influence the outcome of implant treatment and should be thoroughly evaluated during treatment planning.
- •
Practitioners treating patients with systemic metabolic disorders, such as diabetes or osteoporosis, those undergoing radiation therapy, and those who smoke, should follow a 2-staged approach for optimal implant outcome.
Introduction
The clinical effectiveness of the osseointegration concept introduced by Brånemark and colleagues in the 1960s has revolutionized the clinical practice of dentistry. Dental implants are now the preferred line of treatment for the replacement of missing teeth. Additionally, implant-supported full-mouth prostheses are a good treatment option for patients who are completely edentulous, achieving a comprehensive and functional oral rehabilitation. Although endosseous implants have a predictable outcome and long-term success, they sometimes fail. Several clinical studies have recognized risk factors that may lead to implant failure. Implant failures are categorized as primary (early), when the body is unable to establish osseointegration, or secondary (late), when the body is unable to maintain the achieved osseointegration and a breakdown process results. Implant failures also are classified on the basis of the time of prosthesis placement; in this classification, early implant failure usually occurs before the prosthesis is placed, and late implant failure is associated with functional loading after the placement of the prosthesis. Because there are no known noninvasive methods for evaluating the extent of osseointegration, the factors associated with both early and late failures may coexist for a particular implant. Additionally, these factors can be difficult to interpret individually. In a retrospective study, Friberg and colleagues followed 4641 Brånemark dental implants from stage 1 surgery to the completion of the prosthetic restoration. They found that maximal fixtures failed for completely edentulous maxillae with poor bone quality. They also found that some fixtures were not mobile at the abutment connections but were mobile just before the prosthesis was placed. The reason that the fixture gave a false impression of initial stability was that, although the surgeon embedded the implants tightly into the bone, the bone in which the fixture was embedded was remodeled by resorption during the progression of the healing phase. Thus, implant mobility was evident, and the implant failed. Several local and systemic factors, such as lack of primary stability, surgical trauma, and existing periodontal infection, may play an important role in hindering the normal process of bone healing around implants and can subsequently lead to early implant failure. On the other hand, provisional overload and microbially induced peri-implant diseases are associated with late implant failure.
The process of osseointegration between the host’s bone tissue and the implant is the key to the success of the implant. The term osseointegration has several definitions. Albrektsson and colleagues defined it as “a direct structural and functional connection between bone and the surface of a load-bearing implant.” Branemark definition, which is based on macroscopic and microscopic biology from a medical point of view, is “close approximation of new and reformed bone and the fixture together with surface irregularities so that there is no interposition of connective or fibrous tissue at light microscopic level. Thus, a direct structural and functional connection, capable to carrying normal physiologic loads without extensive deformation and initiation of rejection.”
Outcome of the dental implant: success or failure
Several reports have evaluated the successful outcome of dental implants. The term success means attainment of the desired aims. The criteria for successful implants proposed by Albrektsson and colleagues were based on clinical and radiographic evidence of osseointegration, and this criterion is presently most widely accepted. Furthermore, several additions to the criteria of Albrektsson and colleagues have been recently proposed for evaluating successful implants: these additions include clinical function, esthetics, patient satisfaction, radiographic evidence of minimal bone loss, stability of the prosthesis, absence of peri-implant soft tissue infection, and lack of implant mobility and pain. If an implant does not meet all of the criteria for a successful implant, it is instead considered a surviving implant.
On the other hand, implant failure occurs when an implant fails to achieve its function. Usually, failure to attain osseointegration is considered an implant failure. A failed implant must usually be removed. Esposito and colleagues established 4 categories of implant failure based on the osseointegration concept. The first category, biological failure, includes early or primary failure (before loading) and late or secondary failure (after loading). Early or primary failure occurs when osseointegration is not achieved during the initial normal bone-healing process. Late or secondary failure occurs when achieved osseointegration is not sustained. The second category, mechanical failure, is associated with fracture of implants or implant-related structures. The third category, iatrogenic failure, occurs when an implant violates important anatomic neurovascular structures and must be removed. The final category, adaption failure, involves patient-related factors, such as lack of satisfaction, poor esthetic qualities, and the patient’s psychological state.
The most crucial finding related to implant failure is the presence of peri-implant radiolucency and implant mobility. Loss of osseointegration is sometimes characterized by a fibrous connective tissue capsule, called fibro-osseous integration, which cannot withstand the normal physiologic load. Esposito and colleagues stress the difference between failed and failing implants: a failing implant can be saved if it is detected early, whereas a failed implant cannot be saved and must be removed.
Some of the most common causes of implant failure at an early stage are surgical trauma (overheating, inexperienced surgeon), bacterial contamination (failure to maintain aseptic conditions during implant placement, poor oral hygiene status), delayed wound healing (host-related), and early loading of the implants. Esposito and colleagues also reported that the epidemiology of early implant loss is associated with various implant systems, anatomic locations, and several other host-related factors. Reports in the dental literature suggest that the incidence of early implant failure ranges from 0.7% to 2.0%. Implant failure is twice as common among completely edentulous patients as among partially edentulous patients. Both early and late implant failures appear to occur approximately 3 times more frequently in the maxilla than in the mandible.
Introduction
The clinical effectiveness of the osseointegration concept introduced by Brånemark and colleagues in the 1960s has revolutionized the clinical practice of dentistry. Dental implants are now the preferred line of treatment for the replacement of missing teeth. Additionally, implant-supported full-mouth prostheses are a good treatment option for patients who are completely edentulous, achieving a comprehensive and functional oral rehabilitation. Although endosseous implants have a predictable outcome and long-term success, they sometimes fail. Several clinical studies have recognized risk factors that may lead to implant failure. Implant failures are categorized as primary (early), when the body is unable to establish osseointegration, or secondary (late), when the body is unable to maintain the achieved osseointegration and a breakdown process results. Implant failures also are classified on the basis of the time of prosthesis placement; in this classification, early implant failure usually occurs before the prosthesis is placed, and late implant failure is associated with functional loading after the placement of the prosthesis. Because there are no known noninvasive methods for evaluating the extent of osseointegration, the factors associated with both early and late failures may coexist for a particular implant. Additionally, these factors can be difficult to interpret individually. In a retrospective study, Friberg and colleagues followed 4641 Brånemark dental implants from stage 1 surgery to the completion of the prosthetic restoration. They found that maximal fixtures failed for completely edentulous maxillae with poor bone quality. They also found that some fixtures were not mobile at the abutment connections but were mobile just before the prosthesis was placed. The reason that the fixture gave a false impression of initial stability was that, although the surgeon embedded the implants tightly into the bone, the bone in which the fixture was embedded was remodeled by resorption during the progression of the healing phase. Thus, implant mobility was evident, and the implant failed. Several local and systemic factors, such as lack of primary stability, surgical trauma, and existing periodontal infection, may play an important role in hindering the normal process of bone healing around implants and can subsequently lead to early implant failure. On the other hand, provisional overload and microbially induced peri-implant diseases are associated with late implant failure.
The process of osseointegration between the host’s bone tissue and the implant is the key to the success of the implant. The term osseointegration has several definitions. Albrektsson and colleagues defined it as “a direct structural and functional connection between bone and the surface of a load-bearing implant.” Branemark definition, which is based on macroscopic and microscopic biology from a medical point of view, is “close approximation of new and reformed bone and the fixture together with surface irregularities so that there is no interposition of connective or fibrous tissue at light microscopic level. Thus, a direct structural and functional connection, capable to carrying normal physiologic loads without extensive deformation and initiation of rejection.”
Outcome of the dental implant: success or failure
Several reports have evaluated the successful outcome of dental implants. The term success means attainment of the desired aims. The criteria for successful implants proposed by Albrektsson and colleagues were based on clinical and radiographic evidence of osseointegration, and this criterion is presently most widely accepted. Furthermore, several additions to the criteria of Albrektsson and colleagues have been recently proposed for evaluating successful implants: these additions include clinical function, esthetics, patient satisfaction, radiographic evidence of minimal bone loss, stability of the prosthesis, absence of peri-implant soft tissue infection, and lack of implant mobility and pain. If an implant does not meet all of the criteria for a successful implant, it is instead considered a surviving implant.
On the other hand, implant failure occurs when an implant fails to achieve its function. Usually, failure to attain osseointegration is considered an implant failure. A failed implant must usually be removed. Esposito and colleagues established 4 categories of implant failure based on the osseointegration concept. The first category, biological failure, includes early or primary failure (before loading) and late or secondary failure (after loading). Early or primary failure occurs when osseointegration is not achieved during the initial normal bone-healing process. Late or secondary failure occurs when achieved osseointegration is not sustained. The second category, mechanical failure, is associated with fracture of implants or implant-related structures. The third category, iatrogenic failure, occurs when an implant violates important anatomic neurovascular structures and must be removed. The final category, adaption failure, involves patient-related factors, such as lack of satisfaction, poor esthetic qualities, and the patient’s psychological state.
The most crucial finding related to implant failure is the presence of peri-implant radiolucency and implant mobility. Loss of osseointegration is sometimes characterized by a fibrous connective tissue capsule, called fibro-osseous integration, which cannot withstand the normal physiologic load. Esposito and colleagues stress the difference between failed and failing implants: a failing implant can be saved if it is detected early, whereas a failed implant cannot be saved and must be removed.
Some of the most common causes of implant failure at an early stage are surgical trauma (overheating, inexperienced surgeon), bacterial contamination (failure to maintain aseptic conditions during implant placement, poor oral hygiene status), delayed wound healing (host-related), and early loading of the implants. Esposito and colleagues also reported that the epidemiology of early implant loss is associated with various implant systems, anatomic locations, and several other host-related factors. Reports in the dental literature suggest that the incidence of early implant failure ranges from 0.7% to 2.0%. Implant failure is twice as common among completely edentulous patients as among partially edentulous patients. Both early and late implant failures appear to occur approximately 3 times more frequently in the maxilla than in the mandible.
Histomorphology of implant failure
To better understand the mechanism of failure of osseointegrated dental implants, Esposito and colleagues performed histomorphologic analysis of the tissue surrounding 20 failed implants. Four of 20 implants were retrieved by a trephined bur, and the rest were gently unscrewed. Retrieved failed implants fell into 3 groups: group 1, implants failed before abutment connection; group 2, implants failed at the abutment connections; group 3, implants failed after abutment connection. No evidence of bacterial colonization was found in any specimen, but the presence of erythrocytes between implant and bone was a common finding. Failed implants in group 1 exhibited clinical symptoms of infection; the main histologic findings included well-vascularized connective tissue with a small number of inflammatory cells. Furthermore, bone tissue fragments were embedded in the connective tissue. This finding may indicate the situation in which the host cannot regenerate new bone because of surgical trauma. Failed implants in group 2 exhibited no clinical symptoms. There was no direct contact between bone and implant. Histologically, 2 patterns were evident, a finding indicating various mechanisms of failed osseointegration. In the first pattern, the implant was surrounded by dense connective tissue fibers with collagen fiber bundles and elongated fibroblasts as the major component. The second pattern consisted of several layers of nonkeratinized epithelial cells connected by desmosomes and the predominant infiltration of inflammatory cells, such as polymorphonuclear leukocytes, macrophages, and plasma cells. Failed implants in group 3, which were retrieved after abutment connections failed, exhibited variations with regard to the tissue surrounding the implants. In general, there was evidence of mineralized bone; however, it was separated from the implant connective tissue or a layer of nonmineralized bone. Taken together, the results of these histologic studies seem to provide in-depth knowledge about the mechanism of failing osseointegrated implants: these failures seem to be caused by a combination of traumatic and infective factors.
Factors contributed to implant failure
In general, several factors can either directly or indirectly contribute to implant failure and can be broadly classified as internal or external factors ( Fig. 1 ).
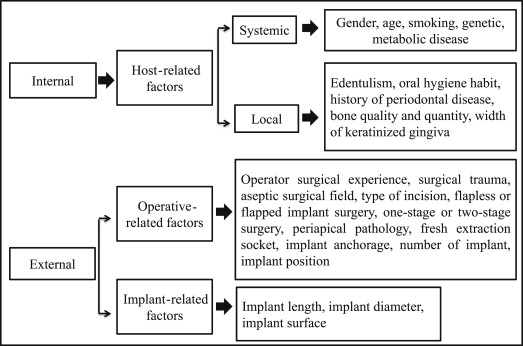
Internal Factors
This classification describes host-related systemic or local factors that may contribute to implant failures.
Host-related factors
Systemic factors
Gender and age
It is unclear how a patient’s gender and age can affect the success of oral implants. An experimental study involving rats demonstrated that the rate and amount of new bone formation surrounding hydroxyapatite-coated implants decrease as patients age. Also, several studies have shown that women are more prone to implant failure than men. Moy and colleagues reported that the risk of implant failure was 2.55 times higher among menopausal women undergoing hormone replacement therapy than among younger women. Another study reported statistically lower implant success rates (78.1%, P <.02) for older patients (>60 years). Although these factors may suggest that the risk of implant failure is higher for women than for men because of menopause and osteoporosis, some studies have shown that the rate of implant failure is higher among men because men tend to smoke more and to have poorer oral hygiene habits. On the other hand, many reports have found no direct evidence indicating that patients’ sex is a potential risk factor for implant failure.
Smoking
Clinical and scientific studies have stressed that smoking has a negative effect on the survival of implants. Smoking is considered an important risk factor for implant failure and is covered in more detail in the article discussing systemic and environmental risk factors for dental implant failure.
Genetics
Although several studies have assessed the genetic polymorphism of the host, it is unclear whether genetic factors are directly related to a patient’s susceptibility to implant failure. Genetics is covered in more detail in systemic and environmental risk factors for dental implant failure.
Metabolic disease
Metabolic disease can directly influence the healing process by affecting bone metabolism (bone remodeling). For patients with systemic conditions, such as diabetes, osteoporosis, hyperparathyroidism, or a history of radiation therapy, clinicians should follow the conventional 2-staged implant system, which provides sufficient time for tissue healing before implant loading occurs. This topic is covered in more detail in systemic and environmental risk factors for dental implant failure.
Local factors
Edentulism
As the patient’s age increases, the patient’s need for periodontal and prosthetic rehabilitation also increases. Furthermore, several studies have reported higher success rates for implant-supported overdentures (>90%) and an improved quality of life among completely edentulous patients. The cost associated with implant-stabilized prostheses sometimes is lower than that associated with alternative treatment options, such as fixed crowns or bridge prostheses. Because the demand for implant-supported overdentures is increasing, it is important that clinicians discuss all of the treatment options available to elderly patients. A common finding among aging patients is an atrophic alveolar ridge, which may limit treatment planning by requiring the insertion of shorter dental implants or the incorporation of procedures such as guided bone regeneration and sinus lift. Several studies have shown that the likelihood of success is lower for short dental implants than for regular dental implants. A morphometric study involving dry edentulous and dentulous mandibles of adult humans found that the presence of some natural teeth significantly influences the dimensions and the anatomy of the mandible. For the reasons discussed previously, the possibility exists that implant outcome also may depend on the presence or absence of dentition. Esposito and colleagues reported that the implant failure rate for subjects with partially edentulous ridges was approximately half that for subjects with completely edentulous ridges. Hultin and colleagues reported that implant failure was more common among completely edentulous patients with implant-supported fixtures than among partially edentulous patients with implants and teeth in the same jaw. There was no significant difference between the survival rates of implants placed in edentulous or partially edentulous jaws. Recently published reports offer limited information about patients’ edentulous status as a factor determining implant success. Future longitudinal studies should evaluate the association between implant success and patients’ edentulous status.
Oral hygiene status
Increased formation of oral biofilm can be expected when a patient has poor oral hygiene. Unlike the natural tooth, dental implants have no periodontal ligament between their surface and the alveolar bone. Thus, microbial plaque can adhere more easily to the rough surface of the implant and can elicit an inflammatory process. If the inflammatory process around the implant is not controlled, it can lead to progressive bone loss and, ultimately, to implant failure. Lindquist and colleagues followed patients with implant-supported fixed mandibular prostheses for 6 years and found that patients with poor oral hygiene and parafunctional habits (jaw clenching) experienced substantial bone loss. A 10-year longitudinal study found that patients who smoked and had poor oral hygiene exhibited significantly ( P <.001) more marginal bone loss than did patients who did not smoke. Likewise, Kourtis and colleagues found that the implant failure rate is higher among patients with unsatisfactory oral hygiene than among those with good oral hygiene ( Fig. 2 ).
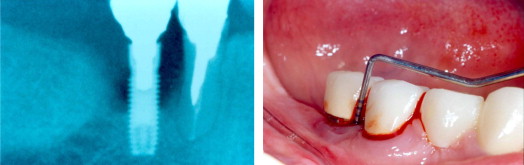
History of periodontal disease
Like periodontitis, peri-implant infection may take years to progress. Therefore, it is not unlikely that peri-implant disease and periodontal disease share common risk factors. The dental literature provides sufficient evidence to indicate that patients with a history of periodontal disease (chronic and aggressive periodontitis) may have an increased susceptibility to peri-implant diseases because of the host’s immune response.
Schou and colleagues reported that the incidence of peri-implant marginal bone loss and peri-implantitis is significantly higher among patients who have already lost teeth because of periodontitis. Additionally, the survival of the suprastructure, bone tissue, and implant was not different in patients with periodontitis and healthy subjects. A recent retrospective study evaluated the outcome of peri-implantitis when patients with severe periodontitis were treated with flap surgery with osteoplasty (47% of cases) or with a few regenerative procedures (20% of cases). Poor treatment outcome was reported for patients with severe periodontitis; this finding suggests the importance of good periodontal and oral health for the success of peri-implant therapy. In contrast, Karoussis and colleagues reported no statistically significant difference in implant outcome between patients with a history of chronic periodontitis and periodontally healthy subjects. However, those researchers also found that, compared with patients with chronic periodontitis, periodontally healthy subjects exhibited shallower probing pockets, less peri-implant marginal bone loss, and fewer incidences of peri-implantitis.
Several reports have suggested that the implant survival rate is poorer and that attachment loss and bone loss are greater among patients with generalized aggressive periodontitis (GAP) than among patients with chronic periodontitis or healthy patients. A recent systematic review and meta-analysis found that the placement of oral implants is a good treatment option for a patient with history of GAP and that the survival outcome is similar for healthy patients and for patients with chronic periodontitis. However, the study also found that the failure rate for dental implants is significantly higher (risk ratio, 4) for patients with GAP than for healthy patients or for those with chronic periodontitis (risk ratio, 3.97) ( Fig. 3 ).
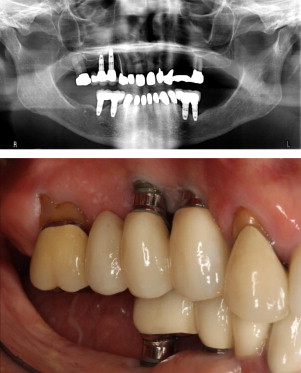
Bone quality and quantity
Published studies have found that failure rates are much higher when the quality and quantity of bone are insufficient at the implant site. Attaining primary stability is a prerequisite for successful osseointegration, and accomplishing primary stability requires adequate bone quantity and density. Lekholm and Zarb developed 4 categories classifying the quantity and quality (shape) of the jawbone to assist in the treatment planning of oral implants: Type I, homogeneous compact bone; Type II, a dense core of trabecular bone surrounded by a layer of thick compact bone; Type III, a dense core of trabecular bone of favorable strength surrounded by a layer of thin cortical bone; and Type IV, a very thin layer of cortical bone with low-density trabecular bone in the center. The investigators also categorized bone quantity (shape): Type A, alveolar bone that is not resorbed; Type B, alveolar bone with some resorption; Type C, complete resorption of the basal bone; Type D, some resorption of the basal bone; and Type E, severe resorption of the basal bone. Various areas of the jawbone have various levels of bone quality. In general, the cortical thickness and density of the mandibular bone are higher than those of the maxillary bone. The posterior segments of both jaws exhibit a marked reduction in cortical bone and increased porosity of the trabecular bone. As a result, implant success rates are higher for mandibular implants, and failure rates are higher in the posterior regions of the jaws. Type 3 or 4 bone quality in the jawbone is associated with high implant failure rates ( Fig. 4 ). Poor bone quantity (inadequate bone height or width) can ultimately lead to the loss of osseointegration because of the limited availability of bone for implant placement. Studies have reported that failure rates are higher for implants placed in Type IV bone. Lindh and colleagues emphasized that the term bone quality is not synonymous with the term bone density . Additionally, these terms refer to different entities: bone quality encompasses not only the mineral content (matrix property and bone density) of the bone but also its structural component (skeletal size, architecture, and 3-dimensional orientation of the trabecular). Furthermore, advanced resorption of the residual ridge of both the maxilla and the mandibular alveolus precludes the placement of longer implants with greater stability because of the proximity of the anatomic structures (maxillary sinus or nasal cavity and mandibular nerve canal). Thus, achieving primary stabilization and a successful outcome for oral implants placed in the jawbone depends primarily on the quality and quantity of bone.
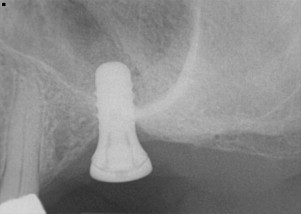
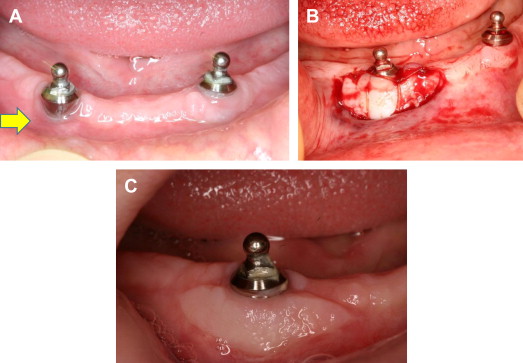
Width of keratinized gingiva
The width of the attached gingiva varies from patient to patient and can be different for different teeth in the same patient. The effect of the presence or absence of adequate keratinized gingiva around dental implants on the success or failure of an implant is a controversial topic. Clinical studies have found that the lack of keratinized or attached mucosa does not compromise the health of peri-implant soft or hard tissues. On the other hand, one study has shown that dental implants that lack adequate keratinized or attached mucosa exhibit more plaque accumulation and mucosal inflammation than implants with adequate keratinized or attached mucosa. However, this study found no correlation between the amount of attached mucosa and implant survival. The presence of keratinized mucosa is strongly correlated with optimal mucosal health and can help prevent infection around implants. Furthermore, a lack of keratinized mucosa is associated with crestal bone loss of 2 mm or more. Therefore, many clinicians believe that the creation of keratinized peri-implant mucosa with sufficient width and thickness is required for preventing implant failure ( Fig. 5 ).

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


