Introduction
Juvenile idiopathic arthritis in temporomandibular joints (TMJs) is often treated with intra-articular steroid injections, which can inhibit condylar growth. The purpose of this study was to compare simvastatin (a cholesterol-lowering drug that reduces TMJ inflammation) with the steroid triamcinolone hexacetonide in experimental TMJ arthritis.
Methods
Joint inflammation was induced by injecting complete Freund’s adjuvant (CFA) into the TMJs of 40 growing Sprague Dawley rats; 4 other rats were left untreated. In the same intra-articular injection, one of the following was applied: (1) 0.5 mg of simvastatin in ethanol carrier, (2) ethanol carrier alone, (3) 0.15 mg of triamcinolone hexacetonide, (4) 0.5 mg of simvastatin and 0.15 mg of triamcinolone hexacetonide, or (5) nothing additional to the CFA. The animals were killed 28 days later, and their mandibles were evaluated morphometrically and with microcomputed tomography.
Results
The analysis showed that the TMJs subjected to CFA alone had decreased ramus height compared with those with no treatment ( P <0.05). Groups that had injections containing the steroid overall had decreases in weight, ramus height, and bone surface density when compared with the CFA-alone group ( P <0.0001). Groups that had injections containing simvastatin, however, had overall increases in weight ( P <0.0001), ramus height ( P <0.0001), condylar width ( P <0.05), condylar bone surface density ( P <0.05), and bone volume ( P <0.0001) compared with the groups receiving the steroid injections, and they were not different from the healthy (no treatment) group.
Conclusions
Treatment of experimentally induced arthritis in TMJs with intra-articular simvastatin preserved normal condylar bone growth.
Highlights
- •
Experimental TMJ arthritis reduces ramus height in growing rats.
- •
Steroid injections in experimental TMJ arthritis further reduce ramus height and bone quality.
- •
Statin injections in experimental TMJ arthritis preserve normal condylar bone growth.
Juvenile idiopathic arthritis (JIA) is a term that encompasses all forms of arthritis of unknown cause that begin before 16 years of age and last more than 6 weeks at a time. A study evaluating the occurrence as well as the clinical signs and symptoms of temporomandibular joint (TMJ) involvement in patients who were diagnosed with JIA reported that of 97 children evaluated, 45% had TMJ involvement. Using more sensitive contrast-enhanced magnetic resonance imaging (MRI), the authors of another study found that 13 of 15 children with newly diagnosed JIA had TMJ inflammatory activity. From an orthodontic perspective, TMJ arthritis may cause limitations in sagittal and vertical mandibular growth that can result in micrognathia and anterior open bite, and many patients require orthodontic treatment or orthognathic surgery to correct the deformities that result from the arthritic destruction of the joint or associated growth disturbances. Since it can be a financial and an emotional hardship for these patients and their families, a method of treatment that can alleviate the damage in the TMJs of patients with JIA is needed.
Well-placed intra-articular injections of corticosteroids have proven to be successful in the management of TMJ arthritis in patients with JIA. A recent systematic review by Stoustrup et al found that intra-articular TMJ corticosteroid injections were successful in reducing inflammation and orofacial symptoms in patients with JIA, but evidence is lacking to prove that the anti-inflammatory properties of the corticosteroids improve maximal incisal opening or normalize or improve mandibular growth in these patients. Two articles that were included in this systematic review found no improvement in mandibular asymmetry or micrognathia after corticosteroid intra-articular injections. Animal studies in rabbits have found that intra-articular injections of corticosteroids caused a decrease in mandibular growth; based on this evidence, intra-articular corticosteroid injections would not be appropriate in growing patients.
A drug that can decrease inflammation and not have a harmful effect on condylar bone growth would be ideal for JIA patients experiencing TMJ arthritis. Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors well known for treatment of hypercholesterolemia, have been found to also have anti-inflammatory properties. Previous studies, involving rats with experimentally induced distal limb joint inflammation, found that when these rats were treated with a subcutaneous injection of simvastatin, early and late joint inflammation was reduced. Most recently, a study in our laboratory found that when rat TMJs were injected with 0.5 mg of simvastatin and an inflammatory agent (complete Freund’s adjuvant [CFA]) was used to induce inflammation, inflammation was greatly reduced when compared with rats that were injected with CFA and the carrier agent alone or CFA and a steroid. Currently, no studies have assessed the growth and morphology changes of an inflamed condyle treated with simvastatin.
This study was designed to evaluate the growth and morphologic changes associated with rat TMJ arthritis treatment with corticosteroid vs simvastatin. To create a model that closely represents JIA as it occurs in humans, CFA was injected into the right TMJs of rats as previously described by George et al. Simultaneously with the TMJ injections of CFA, simvastatin and corticosteroid, either in combination or separately, were locally applied. The hypothesis was that there would be differences among groups relative to condylar growth and bone quality.
Material and methods
Forty-four 3-week-old juvenile male Sprague Dawley rats (Harlan Teklad, Madison, Wis) were allowed to acclimate 1 week before the first procedure. All animals were treated and housed in the University of Nebraska Medical Center College of Dentistry Animal Facility under the auspices of the Animal Care and Use Committee (IACUC # 10-070-FC).
CFA (50 μg of heat-killed Mycobacterium tuberculosis in 50 μL of paraffin oil) was injected into the rats’ right TMJs to induce inflammation based on the protocols described by George et al and Spears et al, showing inflammation lasting for 4 to 6 weeks.
The rats were divided into 6 groups and given unilateral (right side) TMJ intra-articular injections of CFA, 0.5 mg of simvastatin in ethanol, ethanol alone, or 0.15 mg of triamcinolone hexacetonide in combinations outlined in the Table . The injections of CFA and the treatment drugs were given in the same injection to standardize the doses near the head of the condyle and to minimize injection trauma. The left TMJs in all groups received no treatment.
| Group | Rats (n) | Week 0 | Week 4 |
|---|---|---|---|
| 1 | 8 | CFA + 0.5 SIM | Killed |
| 2 | 8 | CFA + EtOH | Killed |
| 3 | 8 | CFA + 0.15 TH | Killed |
| 4 | 10 | CFA + SIM + TH | Killed |
| 5 | 6 | CFA | Killed |
| 6 | 4 ∗ | No treatment | Killed |
Ethanol acted as the carrier for the simvastatin to allow the simvastatin to dissolve, and sterile water was the carrier for the triamcinolone hexacetonide as per the protocol for human TMJ injections. All intra-articular injections were delivered with an insulin syringe with a 30-gauge needle (Becton Dickson, Franklin Lakes, NJ). The doses were based on previous studies showing that 1 intra-articular injection of 0.5 mg of simvastatin caused a significant decrease in histologic condylar inflammation in rats when compared with treatment with triamcinolone hexacetonide or no treatment. The triamcinolone hexacetonide dose of 0.15 mg (1.0 mg/kg) was based on previous rabbit studies and is roughly equivalent to the human dose.
Anesthesia was induced by placing the rats into an incubation chamber with 1% to 4% isofluorane/100% oxygen (1-3 L/minute). The depth of anesthesia was based on the depth and pattern of breathing and the animal’s response to the pedal withdrawal reflex. After removal from the chamber, the rat’s nose was placed into a nose cone, and 0.5% to 2% isofluorane/100% oxygen (0.5-1 L/minute) was used to maintain anesthesia during the injections.
At week 4, all animals were killed by carbon dioxide asphyxiation. The mandible was separated from the rest of the skull and placed in 10% formalin for storage before morphometric and microcomputed tomography measurements.
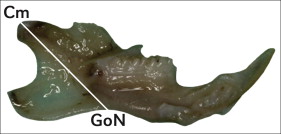
Morphometric measurements were taken of each side of all rat mandibles after defleshing the specimens and before microcomputed tomography evaluation using a digital caliper (Mitutoyo, Takatsu, Japan). Overall ramus height and growth were estimated by measuring from the condylar midpoint (Cm, the midpoint of the condylar head located at the bisection of the absolute sagittal length of the upper margin) to the gonial notch (GoN, the most superior point of the convexity of the lower border of the mandible) ( Fig 1 ).
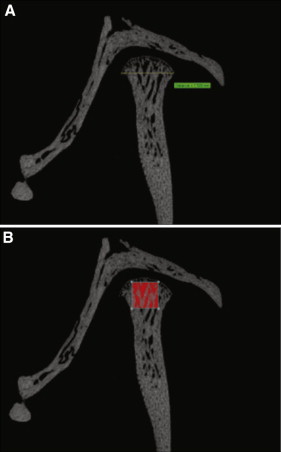
It has been shown that inflammation in the TMJ leads to morphologic changes that can be seen in tomograms and 3-dimensional imaging. The mandibles were scanned with a high-resolution system (model 1172; Skyscan, Aartselaar, Belgium). The x-ray source was set at a voltage of 44 kV with current at 226 μA and a fixed exposure time of 610 milliseconds. The resolution was 8.7 μm using a camera pixel size of 1336 × 2000 with a 0.5-mm aluminum filter. Five frames were averaged for each rotation with a rotation step of 0.7° after an angle of 180°. Three-dimensional reconstructions were performed by the system reconstruction software (NRecon; Skyscan). All specimens were aligned on 3 axes (sagittal, coronal, transaxial) until the head of the condyle from each view was centered in each frame. The coronal or sagittal views, designated by the Skyscan software, were used to obtain the measurements. The raw images reconstructed by the program were enhanced with a histogram. After defining the region of interest in the bone, half-tone binary images were displayed, and the upper and lower values on a histogram were adjusted on the specimens to capture the bony morphology of the TMJ as areas with brightness (white) within the range of the binary threshold selection ( Fig 2 ). The largest measurement for condylar width was recorded to estimate condylar growth ( Fig 2 , A ). This method is similar to that used by Kuroki et al. Bone volume and bone surface density measurements were taken in a volume of interest in the condyle, based on the method by Jiao et al, measuring 0.784 × 0.784 × 0.784 mm ( Fig 2 , B ). This location and size were based on the largest cube that could be fitted into the head of all condylar specimens with the upper edges touching the superior border of the condylar head, and in the closest proximity to the CFA and drug injections.
Statistical analysis
An average of 8 rats per group was proposed in this study based on histologic detection of a decrease in inflammation with administration of a 0.5-mg simvastatin injection, and a preliminary power analysis indicated that 5 to 8 rats would be necessary to show a ≥30% increase in mandibular bone growth 80% of the time at the 5% level of significance with varying doses of simvastatin. Differences in the actual numbers in each group were because (1) 2 extra animals received from the vendor were arbitrarily added to the CFA, simvastatin, and triamcinolone hexacetonide group, (2) the CFA-only group was studied during a different experimental session and started with 7 rats, and (3) 1 rat from the CFA group could not be evaluated for condylar measurements because of a condylar fracture during specimen harvest. All group numbers were within the range of the original power analysis. The specimens were coded by animal number and side (right or left) and measured by 1 examiner (C.H.) without knowledge of group designation. The same examiner repeated these measurements 2 weeks later on 10% of the original specimens to confirm the reliability of the measurements. A mixed model repeated measures analysis was used to determine whether the variance caused by animal was significantly greater than zero, indicating that between-animal measurements varied significantly more than within-animal measurements. Analysis of variance was used for intergroup comparisons. The results were considered significant when P values were ≤0.05.
Results
The rats’ mean weight (±standard deviation) at week 0 (baseline) was 105.7 ± 5.7 g. There were no statistically significant differences among groups. The weights of the animals at week 4 showed increases in all groups because they were growing animals. Weight gains for the rats treated with CFA and steroid (123.3 ± 32.8 g) and the combination of CFA, simvastatin, and steroid (116.6 ± 24.4 g) were statistically less ( P <0.01) at week 4 than all other therapeutic groups (CFA and simvastatin, 161.9 ± 7.3 g; CFA and ethanol, 160.1 ± 12.7 g; CFA only, 158.2 ± 16.6 g; no treatment, 152.8 ± 20.9 g). No other significant differences were noted. No animals died prematurely, all animals tolerated the procedures well, and no groups showed abnormal signs of stress (not eating, inactivity, piloerection, scratching at the jaw). Porphyrin staining was seen in all groups for the first 2 or 3 days after the TMJ injections.
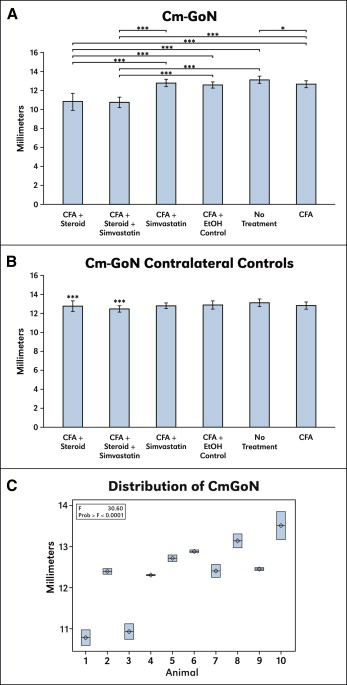
Ramus height measurements (Cm-GoN) are summarized in Figure 3 , A . Repeated measures of 10% of the specimens with a mixed-model repeated measures analysis showed that the estimated variance among animals (0.75 ± 0.36) was much greater than the residual estimate (0.05 ± 0.02) (F value, 30.60; P <0.0001), indicating high reproducibility of ramus height ( Fig 3 , C ). Differences in the linear measurements of ramus heights between groups showed that the CFA and steroid group and the CFA, simvastatin, and steroid combination group were statistically smaller than all other groups. In the CFA-alone group, ramus height was also statistically smaller than in the no-treatment group. When the contralateral untreated TMJs were evaluated, no statistical differences were found among the groups ( Fig 3 , B ). However, in both the CFA and steroid control group and the CFA-combination control group, ramus heights were greater than their contralateral treatment sides.
Microcomputed tomography measurements that showed statistically significant differences among groups were condylar width at the greatest point (CWGREAT), bone volume in the condyle (BONEVC), and bone surface density in the condyle (BONEDC). Differences in the linear measurements of CWGREAT among groups showed that the CFA and steroid group was statistically smaller than the CFA and simvastatin group and the CFA and ethanol group. The CFA, simvastatin, and steroid combination group was statistically smaller than all other groups except for the CFA and steroid group ( Fig 4 , A ). When the contralateral untreated TMJs were compared, there were no statistical differences among the groups ( Fig 4 , B ). However, the CFA, simvastatin, and steroid combination group and the CFA and steroid group were statistically smaller than their contralateral controls.





