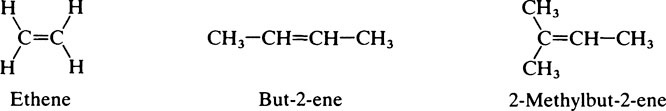From atoms to molecules
Publisher Summary
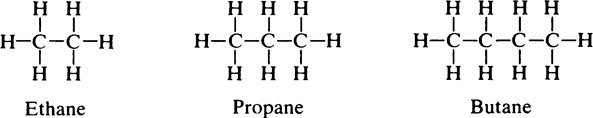
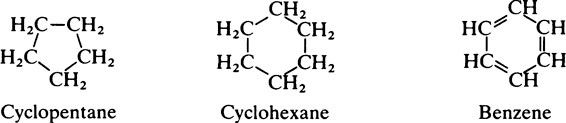
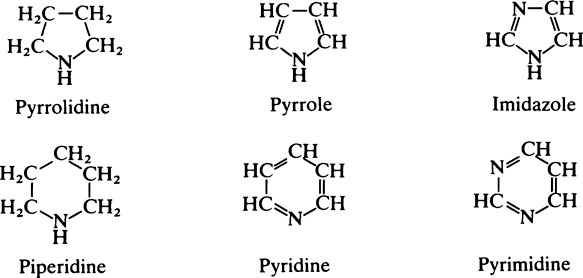
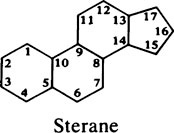
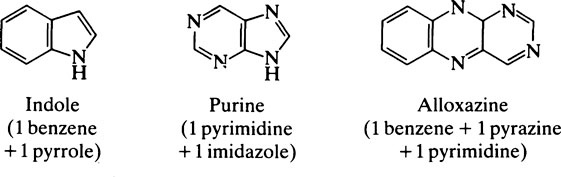
This chapter discusses the macromolecules that characterize living organisms. Life is based on carbon compounds and is only possible on account of their variety and complexity. As carbon atoms can form stable covalent bonds with other carbon atoms, chains and ring structures can be built up. The parent substances for all other organic compounds are the hydrocarbons that contain hydrogen as the only other element besides carbon. Their molecules contain a number of carbon atoms linked to give either an open chain structure or a closed form containing one or more rings. Certain derivatives of ring hydrocarbons containing five or six carbon atoms and their unsaturated counterparts are biologically important. Benzene, which contains alternate, resonating double bonds, is the parent substance of the aromatic series of compounds. As distinct from benzene and other ring systems that contain carbon as the only ring constituent are a number of heterocyclic ring compounds in which one or more of the carbons is replaced by an oxygen, nitrogen, or sulfur atom. These may be either saturated or unsaturated.
Functional groups
The hydrocarbons and heterocyclic ring compounds described above are rather inert and unreactive but they become more reactive if one or more of their hydrogens is substituted by other groupings, notably those containing oxygen or nitrogen. Biochemically speaking the most important of these functional groups are shown in Table 2.1.
Table 2.1
Some important functional groups
| N containing | O containing | Carbonyl containing | S containing | P containing |
| –NH2 (amino) | –OH (hydroxyl) | –COOH (carboxylic) | –SH (sulphydryl) | –PO4H2 (singly esterified phosphate) |
| =NH (imino) | =CO (carbonyl) | –CONH2 (amide) | –S–S– (disulphide) | –PO4H– (doubly esterified phosphate) |
| –SO3H (sulphonic acid) |

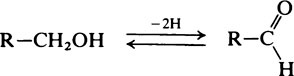
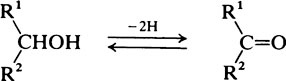
Hydroxyl groups
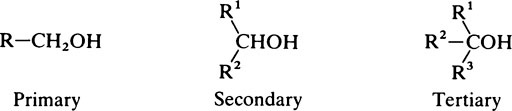
on the C atom which bears the hydroxyl group. Alcohols react with acids to form esters and two important classes of biological substance are compounds of this type, namely the triglycerides and the phosphoric acid esters.
Hydroxyl groups which are directly attached to an aromatic nucleus have rather different properties from aliphatic hydroxyl groups since they can act as proton donors and are therefore weakly acidic as in the case of tyrosine (page 37).
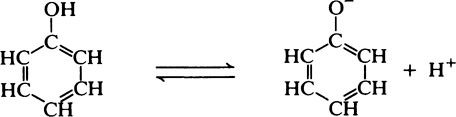
Phenolic substances also resemble the alcohols in their ability to react with acids to form esters.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses