19
Failure of Dental Local Anesthesia
Despite scientific advances, there is, unfortunately, no infallible technique for ensuring local anesthesia in dentistry, and failures generally reflect specific situations such as anatomical variations, pathological abnormalities (e.g. local inflammation), considerable patient anxiety, and failures associated with the dentist. In this chapter, we address the area of failure of dental anesthesia and its causes and examine the means at our disposal to overcome it.
Frequency
It is difficult to quantify how often dental local anesthesia fails because pulpal anesthesia, the most difficult type of anesthesia to achieve, is hampered by various factors.
- Type of stimulus. The pain stimulus differs depending on whether the procedure is obturation (especially if it is not very deep), extraction, incision of soft tissue, or an endodontic procedure in a vital tooth. It is much easier to anesthetize the soft tissues or alveolar process than the dental pulp (Phillips 1943). We believe that the electric pulp meter is the most reliable experimental stimulus for evaluating the efficacy of local pulpal anesthesia since it reaches deep levels and its findings are reproducible. Therefore, in this book, we have selected studies whose results are based on the application of this approach.
- Type of local anesthetic solution. Different outcomes are achieved by varying the concentration of anesthetic and/or vasoconstrictor, as well as by using different local anesthetics or vasoconstrictors. This is particularly important in infiltrative techniques (Annex 21), although generally of little relevance in mandibular block (Annex 24). Therefore, we have selected the two most widely used solutions today: the one we have referred to throughout the book as the standard solution, i.e. lidocaine 2% with epinephrine 1:100 000 (10 μg/ml) (L‐100) or 1:80 000 (12.5 μg/ml) (L‐80), and a potent solution, i.e. articaine 4% with epinephrine 1:100 000 (10 μg/ml) (A‐100).
- The amount of solution administered (in milliliters [ml]). We selected the standard quantities on which current clinical evaluations are based (generally slightly higher than recommended in textbooks) and which are those used in daily clinical practice.
- Area of the mouth. The results are very different for the maxillary and the mandibular arches and are very different for the anterior teeth (canines and incisors) and posterior teeth (molars and premolars). Therefore, we have drawn a distinction between these four areas and have taken the lateral incisor and first molar as a reference.
Table 19.1 summarizes the percentage of failures in healthy teeth that respond to an electric stimulus despite being anesthetized. A study from 2002 in patients receiving dental treatment over 5 years found that treatment was painful in more than 40%. In addition, the pain was moderate or intense in more than half (20% of the total) (Maggirias and Locker 2002). Consequently, dental local anesthesia could be improved.
Consequences of Failure
Failure of dental locoregional anesthesia is important for various reasons:
- Dental treatment cannot be administered.
- Patients lose trust not only in their dentist, but also in modern dental techniques for controlling pain (Kaufman et al. 1984).
- Failure is especially important in children because traumatic experiences (e.g. pain during dental procedures resulting from insufficient anesthesia) generate psychological effects, i.e. patients become very fearful of dental treatment and may develop phobias during adulthood (Molin and Seeman 1970; Kleinknecht et al. 1973; Cohen et al. 1993; Berggren and Meynert 1984).
Table 19.1 Summary of failures.
| Tooth | Anesthetic technique | ml/LAS/time (min) | Failure | Reference | ||
|---|---|---|---|---|---|---|
| Maxillary teeth | ||||||
| Incisors | Buccal infiltration | 1.0/L‐100/5′ | 5% | Annex 21 | ||
| Buccal infiltration | 1.0/A‐100/5′ | 2% | Annex 21 | |||
| First molar | Buccal infiltration | 1.8/L‐100/5′ | 13% | Annex 21 | ||
| 1.8/A‐100/5′ | 5% | Annex 21 (estimated) | ||||
| Mandibular teeth | ||||||
| Incisors | Buccal infiltration | 1.8/L‐100/5–10′ | 45% | Annex 31 | ||
| Buccal infiltration | 1.8/A‐100/5–10′ | 20% | Annex 31 | |||
| Double infiltration | 1.8/L‐100/5–10′ | 10% | Annex 31 | |||
| Double infiltration | 1.8/A‐100/5–10′ | 2% | Annex 31 | |||
| Mandibular block | 1.8/L‐100/10–15′ | 65–70% | Annex 25 | |||
| Mandibular block | 3.6/L‐100/10–15′ | 60–70% | Annex 25 | |||
| First molar | Mandibular block | 1.8/L‐100/10–15′ | 35–40% | Annex 25 | ||
| Mandibular block | 3.6/L‐100/10–15′ | 30–35% | Annex 25 | |||
 |
Mandibular block + complementary buccal infiltration | 1.8/L‐100/10–15′ 1.8/A‐100/5–10′ |
 |
5% | Table 16.5 Chapter 16 |
|
Percentage of failures with the most frequently used techniques in healthy teeth. The lowest percentages of failure are shown in bold. Evaluation is with electrical stimulation.
ml/LAS/time, milliliters/local anesthetic solution/time in minutes; L‐100, lidocaine 2% with epinephrine 1:100 000 (10 μg/ml); A‐100, articaine 4% with epinephrine 1:100 000 (10 μg/ml).
Failures: General Causes
Many failures are associated with patient‐specific situations.
Highly Anxious Patients
As we saw in Chapter 8, around 10% of patients are highly anxious and fear dental treatment. Anxiety reduces the pain threshold and increases the possibility that nonpainful stimuli are interpreted as being painful (Pinkham and Schroeder 1975; Wepman 1978; Woolgrove 1983; Sokol et al. 1985; Van Wijk and Makkes 2008). It is therefore important to bear in mind that local anesthetics are very effective for anesthetizing painful stimuli but are much less effective with sensations of temperature and pressure. In addition, they are poorly effective with the nerve fibers that transmit proprioceptive stimuli (de Jong 1977; Wildsmith 1986). In such patients, it is necessary to follow various steps:
- Inform the patient that he/she should distinguish between “painful” stimuli, which can be easily anesthetized, and sensations of touch, pressure, and temperature, which are more difficult to anesthetize. Thus, we can help the patient to interpret these sensations in two ways:
- Extraorally:
- The dentist can take the patient’s hand by the wrist in his/her own hand, move it from side to side, and ask the patient if he/she notices this and if it hurts. The patient will reply that he/she does notice it but that it does not hurt.
- The dentist presses the patient’s hand with his/her own and asks if the patient notices pressure and if this is painful. The patient will reply that he/she does notice the pressure but that it is not painful.
- The dentist asks the patient if his/her hand is cold or warm. The patient replies that it is warm.
- Finally, the dentist points out to the patient that he/she felt the movement of the hand, the pressure, and the temperature, but no pain. The same will be true of the mouth, that is, the patient will notice sensations (movement, pressure, temperature) but not pain.
- Intraorally. The word “pain” is now taboo, and the euphemism “discomfort” is used instead.
- The dentist shows the patient his/her right hand with the fist closed and the index finger extended and says, “Look at my finger.”
- The dentist then inserts the finger into the patient’s mouth and presses on the injection site and says “Here is where I’ll place the anesthesia. Can you feel where I’m touching you? The patient will reply that he/she does notice it. Observe that the dentist says “place” the anesthesia and not “inject,” which is taboo.
- The dentist says that when the anesthetic is placed in this area, the patient will notice it or perhaps will feel some “discomfort” (euphemism for pain, now a taboo word).
- Extraorally:
- The dentist must exercise great care when administering local anesthetic to minimize the pain of the injection (see sections “Insertion of the Needle” and “Injection”, Chapter 13). In addition, it is advisable to administer a larger quantity of anesthetic.
- If necessary, the patient should receive an anxiolytic or sedative drug, although therapy of this type is beyond the scope of this book.
- If the patient continues to feel pain for any reason during treatment, supplementary techniques can be applied to address failure (see Chapter 18).
However, if the level of anxiety is very high or the patient is phobic (irrational and uncontrolled fear), then he/she must be treated by a psychologist or psychiatrist. Such patients may require psychiatric drugs or general anesthesia, both of which approaches are beyond the scope of this book.
Patients with Drug Addiction and Alcoholism
Patients who are addicted to alcohol or other drugs such as heroin, cocaine, or tranquilizers have a very low tolerance of stress (nervousness) and pain because of psychological and physiological abnormalities affecting the central nervous system (Scheutz 1982; Chemical 1987; Stewart and Finn 1995; Fiset et al. 1997; Lindroth et al. 2003). These patients require greater amounts of anesthesia, and anesthesia fails twice as often as in patients who do not have addictions (Scheutz 1982; Chemical 1987; Stewart and Finn 1995). In addition, they are often difficult to manage and require both medical and psychological treatment.
This group of patients should be treated in the same way as highly anxious patients (see above).
Teeth Affected by Irreversible Acute Pulpitis
The pulp of teeth affected by acute pulpitis (symptomatic irreversible pulpitis) is both inflamed and hypersensitive (called a “hot tooth”). The frequency of failure of local anesthetic is higher, i.e. double or triple that of patients without pulpitis (Table 19.2), given that the tooth is more difficult to anesthetize. In Table 19.2, failure in healthy molars is evaluated based on an electric pulp meter; however, in molars affected by irreversible acute pulpitis, failure is evaluated in endodontic procedures when the pulp chamber is opened and during cleaning because negative electrical stimulation is no guarantee of a painless endodontic procedure in these cases (Dreven et al. 1987; Reisman et al. 1997; Nusstein et al. 1998; Tortamano et al. 2009). It is also important to highlight that the more severe the symptoms are in teeth with pulpitis (more pain), the greater the percentage of failures will be (Aggarwal et al. 2015).
Table 19.2 Percentage of failures after local anesthesia in molars with irreversible acute pulpitis compared with healthy molars.
| Anesthetic technique | ml/LAS/time (min) | Failure healthy molars | Failure molars with pulpitis |
|---|---|---|---|
| Maxillary teeth | |||
| Buccal infiltration | 1.8/L‐100/5–10′ | 13% | 35% |
| “ | 1.8/A‐100/5–10′ | 5% | 25% |
| Mandibular teeth | |||
| Mandibular block | 1.8/L‐100/10–15′ | 35–40% | 70% |
| Mandibular block | 3.6/L‐100/10–15′ | 30–35% | 55% |
| Reference | Table 19.1 | Annex 35 | |
ml/LAS/time, milliliters/local anesthetic solution/time in minutes; L‐100, lidocaine 2% with epinephrine 1:100 000 (10 μg/ml); A‐100, articaine 4% with epinephrine 1:100 000 (10 μg/ml).
Reasons for Failure of Anesthesia in Acute Pulpitis
- Structural abnormalities affecting peripheral nerves. These nerves are affected by inflammation (Kimberly and Byers 1988; Byers et al. 1990; Taylor and Byers 1990; Sorensen et al. 2004), and their thresholds of excitability and of electrolyte exchange in the membrane are altered, thus rendering the membrane hyperexcitable (Brown 1981; Rood and Pateromichelakis 1981). These neurodegenerative changes affect not only the axonal membrane exposed to inflammation, but also the whole nerve pathway, therefore truncal block at some distance from the inflammation also fails (Najjar 1977; Wallace et al. 1985; Luo et al. 2008).
- Local factors:
- Tissue pH in inflamed or purulent areas is lower and may reach 5–6.6 (Schade et al. 1921; de Jong and Cullen 1963) instead of the 7.4 observed in healthy tissue, therefore the acid environment leaves very little free base for the anesthetic to penetrate the cell membrane (Bieter 1936; de Jong 1977; Walton and Torabinejad 1992; Wong and Jacobsen 1992).
- Increase of inflammatory mediators (such as prostaglandins, calcitonin gene‐related peptide, substance P, neurokinin A, neuropeptide Y, vasoactive intestinal polypeptide) that sensitize the sodium channels of the free nerve endings by facilitating depolarization with less intense stimuli (hyperalgesia), therefore local anesthetics are less efficacious for blocking them (Bowles et al. 2003; Lai et al. 2004; Caviedes‐Bucheli et al. 2006).
- Modifications in the sodium channels of the dental pulp lead to a threefold multiplication of the subtype or isoform Nav 1.9 (Wells et al. 2007), which requires 2.5–5 times more anesthetic for the block to be effective (Scholz et al. 1998); six to eightfold of the subtype Nav 1.8 (Renton et al. 2005; Warren et al. 2008) and also the subtype Nav 1.7 (Luo et al. 2008).
- Regional vasodilation favors rapid removal of the anesthetic solution at the affected site as a result of it entering the systemic circulation (Kramer and Mitton 1973; Meechan 1999).
- Tissue pH in inflamed or purulent areas is lower and may reach 5–6.6 (Schade et al. 1921; de Jong and Cullen 1963) instead of the 7.4 observed in healthy tissue, therefore the acid environment leaves very little free base for the anesthetic to penetrate the cell membrane (Bieter 1936; de Jong 1977; Walton and Torabinejad 1992; Wong and Jacobsen 1992).
Approach
- Administer a nonsteroidal anti‐inflammatory drug (NSAID) 45–60 minutes before local anesthesia. The most widely used NSAID is ibuprofen at 400–800 mg, although any NSAID can be used (Annex 35; Modaresi et al. 2006). When NSAIDs cannot be used (e.g. in patients with gastrointestinal ulcer, pregnant women, patients taking oral anticoagulants, aspirin‐sensitive asthmatics), acetaminophen can be administered at 1000 mg, although the outcome is somewhat more modest (Annex 35; Modaresi et al. 2006). In addition, two meta‐analyses have shown that NSAIDs taken 1 hour before the procedure improve anesthesia in mandibular block. This finding was statistically significant (Li et al. 2012; Shirvani et al. 2017).
- Administration of local anesthesia (Annex 35).
- Use potent solutions such as articaine 4% with epinephrine 1:100 000 (10 μg/ml) (A‐100) instead of the standard solution of lidocaine (Annex 35). Three meta‐analyses have demonstrated the superiority of A‐100 over the standard lidocaine solution (L‐100 or L‐80) in patients with irreversible acute pulpitis (Kung et al. 2015; Su et al. 2016; de Geus et al. 2020).
- In maxillary teeth:
- Anterior teeth. Buccal infiltration with 1.8 ml of A‐100 complemented by a small amount administered via the area of the palate. Wait a little longer, 5–10 minutes.
- Posterior teeth. Buccal infiltration with more than 1.8 ml of A‐100 (75% success rate [Annex 35]) and complement with 0.3–0.4 ml via the palate to enhance anesthesia of the palatal roots (Ulusoy and Alacam 2014; Askari et al. 2016).
- In mandibular teeth:
- Anterior teeth. Double infiltration (buccal and lingual) of 1.8–3.6 ml of A‐100. Wait 5–10 minutes for the anesthetic to take effect.
- Posterior teeth. Mandibular block with1.8 ml of A‐100. Wait 5 minutes to ensure that the lower lip is anesthetized. At this point, inject a further 1.8 ml of A‐100 as mandibular block (two cartridges, 3.6 ml, have now been injected) and use complementary anesthesia in the buccal region with a further 1.8 ml of A‐100 (three cartridges have now been injected). Wait 5–10 minutes longer (10–15 minutes in total). It is important to take two aspects into account:
- If the second mandibular block is performed using the Gow‐Gates technique instead of the conventional technique used for the first block, it increases efficacy and the number of teeth anesthetized (Saatchi et al. 2018).
- It is interesting to observe that mandibular block is more painful in these cases (McCartney et al. 2007; Fan et al. 2009; Kreimer et al. 2012; Annex 23), therefore the technique should be performed meticulously.
- Anesthesia with supplementary techniques. If the above approach is insufficient and the anesthetic fails to take effect.
Failure of mandibular block affects the dentine in 30% of cases (pain is felt when the burr reaches the dentine), therefore it is too early to apply the intrapulpal technique and it is necessary to turn to other supplementary techniques (Annex 35).
We can turn to the periodontal ligament technique (PDL) and the intraosseous technique (IO), again using A‐100, which we have used so far (it is important to remember not to mix two local anesthetics at the same point of action, Chapter 5). The initial success with the first injection is greater with the IO technique than with the PDL (85% vs. 65%), although the cumulative effect after the second injection (if the first fails) is very similar (almost 100%) (Annex 35). However, the supplementary technique may sometimes have to be repeated as many as three times (Nusstein et al. 1998). The PDL has the advantage that if a rubber dam is in place, it is not necessary to remove it for administration (Walton and Abbott 1981; Khedari 1982); the advantage of the IO technique is that is has fewer adverse effects (Chapter 18).
In patients with irreversible acute pulpitis, supplementary techniques can be painful because of the high sensitivity of the teeth, even if all the tissue is anesthetized (Nusstein et al. 2003).
It is also noteworthy that other methods are currently being sought, such as inhalation of nitrous oxide, for which data seem promising (Fullmer et al. 2014; Chompu‐inwai et al. 2018).
Finally, remember that endodontic procedures are considered to be successful when the anesthetized teeth do not hurt or only do so minimally, so that the procedure can be performed with the drill able to penetrate the enamel and dentine and reach the pulp chamber (Annex 35). These teeth may subsequently require intrapulpal anesthesia.
Resistance to Local Anesthetics
Cases of resistance to the action of various local anesthetics have been reported. In both medical practice (Miller et al. 1981; Kavlock and Ting 2004) and in dental practice (Beckett and Gilmour 1990), resistance takes the form of short duration of effect or insufficient effect. In dental practice (Beckett and Gilmour 1990) this cannot be attributed to the traditional causes of failure addressed in this chapter, but rather to genetic abnormalities that lead to structural abnormalities in some of the isoforms of the sodium channels (Panigel and Cook 2011; Clendenen et al. 2016). The frequency of resistance is unknown, although it must be low, and in some cases it has been overcome using local anesthetics in which the concentration of the anesthetic component is high (Beckett and Gilmour 1990).
Other Causes of Failure
Other proposed causes of failure include hematoma in mandibular block that could dilute the anesthetic solution in the pterygomandibular space (Traeger 1979), although this seems highly unlikely.
Specific Failures After Maxillary Infiltration
Failure of local anesthesia is less frequent after infiltration in maxillary teeth than after mandibular block. Below, we present the causes and the means to overcome them.
Causes of Maxillary Failure
- Excessive thickening of the maxillary bone cortex. This mainly affects the superior central incisors via the anterior nasal spine (Figure 19.1) if this is very wide and covers the apices. However, such a situation is highly unlikely (Jastak et al. 1995). This situation may also arise in the first molars owing to thickening of the zygomatic crest (Evers and Haegerstam 1981; Roberts and Sowray 1987; Jastak et al. 1995) or thickening of the outer bone plate, which is typical of patients with bruxism.
- Excessive separation of the palatal roots of the molars, premolars, and lateral incisor as they course toward the palate. In the posterior teeth, the distance separating the palatal roots and the buccal roots may be very great. In fact, the maxillary sinus may even lie between the palatal and the buccal roots (Figure 19.2), thus hampering diffusion of the anesthetic solution toward the palatal root (Evers and Haegerstam 1981; Haglund and Evers 1985). Very rarely, the root of the lateral incisor may be inclined toward the palate.
- Accessory innervation via the nasopalatine nerve to the pulp of the incisors and, occasionally, the canines. This branch of the nasopalatine root was proposed initially by Otto Hofer from Vienna (Hofer 1922) and supported, albeit without demonstration, by various authors (Phillips and Maxmen 1941; Cook 1949). In 1943, the Department of Anatomy of the University of Wayne reported having found this branch in dissections (Phillips 1943), although other studies based on dissection did not (Olsen et al. 1955), therefore these contradictory results created an atmosphere of distrust (FitzGerald and Scott 1958; Westwater 1960; Sicher 1950). More recently, it was suggested that fibers of the superior dental plexus can join the nasopalatine nerve right at the nasal floor and reach the apices of the central incisors (Roda and Blanton 1994).
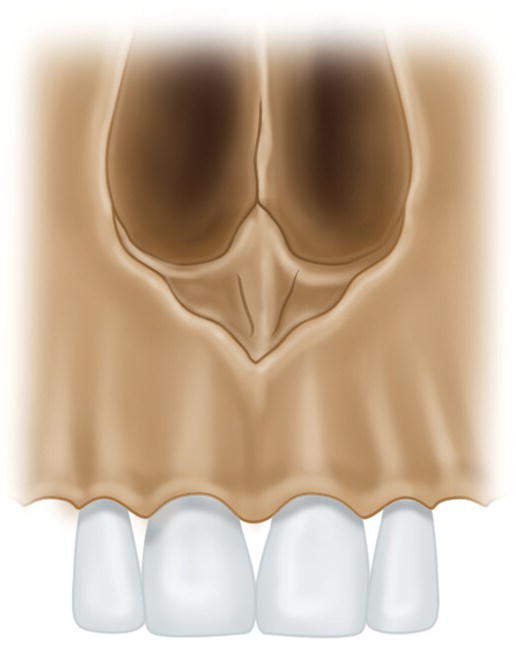
Figure 19.1 Thickening of the anterior nasal spine covering the apices of the maxillary central incisors.
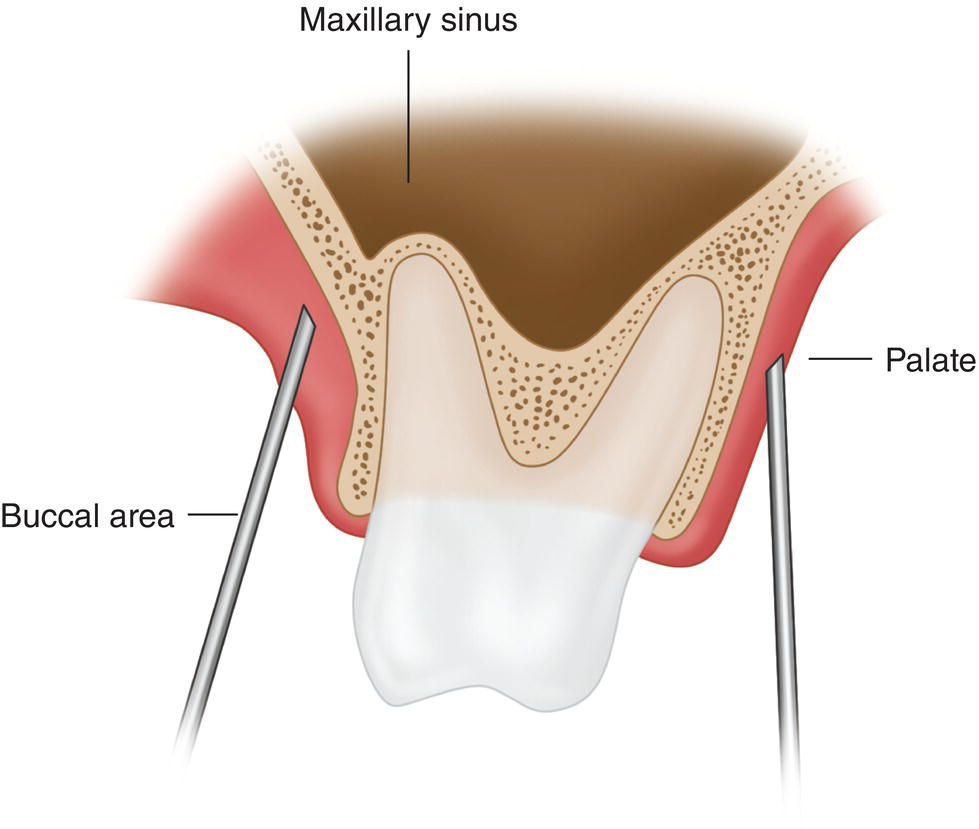
Figure 19.2 Maxillary sinus entering the space between the palatal and buccal roots.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


