In order to develop a rational plan for management of oral and maxillofacial vascular anomalies, clinicians must have a basic understanding of the biology of these lesions and the nomenclature and classification system used to characterize them. Often referred to as birthmarks, vascular lesions are often as misunderstood by the medical community as they are by the lay community. While a standard classification system has been adopted, the medical literature is replete with archaic terminology that persists and continues to be passed on to new generations of clinicians. Outdated terms such as “stork bite,” “angioma simplex,” or the indiscriminately applied “hemangioma” were derived from classification systems that were either descriptive, pathological, or embryological in nature. A biologically based classification system of vascular lesions proposed by Mulliken and Glowacki in 1982 was accepted as the standard classification system by the International Society for the Study of Vascular Anomalies in 1996. This binary system designates vascular lesions as either tumors or malformations based on endothelial cell kinetics and clinical behavior. Vascular malformations are relevant to the daily practice of most oral and maxillofacial surgeons and will be the focus of this chapter. Vascular tumors will be discussed only in the context of differentiating them from malformations.
Etiopathogenesis
The innermost cell layer of hematic and lymphatic vessels, the endothelium, has a relatively slow turnover time. A vascular lesion that demonstrates endothelial hyperplasia (enlargement due to increased cell number), with mitotic figures and short doubling times, is categorized as a vascular tumor . The most commonly encountered vascular tumor (and, for that matter, most common tumor of infancy) is the infantile hemangioma. This tumor generally appears at or shortly after birth (most commonly in the craniofacial area) and rapidly grows for the first year of life, then slowly involutes over the next 5 to 7 years until an end-stage, involuted lesion remains. It occurs more commonly in premature and female neonates, does not occur intraosseously, and while it rarely (<1%) can lead to deformational changes in adjacent bone, it has no direct physiological influence on osseous growth. In turn, a vascular lesion that exhibits a normal rate of endothelial turnover but structural and morphological anomalies due to errors in embryogenesis is termed a vascular malformation . Vascular malformations are always present (though not always seen) at birth, do not proliferate but instead demonstrate slow, relentless expansion, and do not involute. The reader may question this statement, having experienced abrupt changes in the presentation of a long-standing vascular malformation. Trauma, sepsis, hormonal modulation, and changes in blood or lymph flow and/or pressure may indeed temporarily speed up a malformation’s expansion, but this is due to hypertrophy (enlargement due to an increase in size of existing structures) and not hyperplasia. Vascular malformations have equal sex distribution, can occur intraosseously, and in up to a third of the cases produce secondary skeletal changes. This last point is of particular importance to the oral and maxillofacial surgeon.
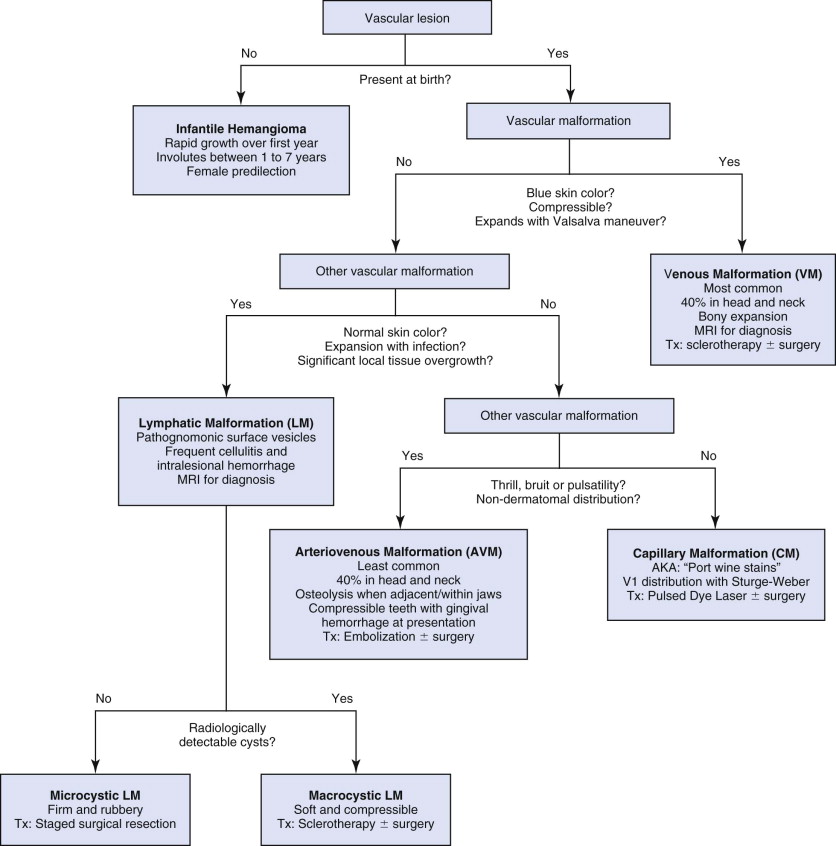
Vascular malformations can usually be differentiated from hemangiomas by clinical exam and history with an accuracy of 90% ( Fig. 111-1 ). In the rare situation where the diagnosis is equivocal, additional studies can be obtained. Histological studies of a biopsy specimen should be obtained, especially if a rare or possibly malignant vascular tumor is suspected. Lawley et al. demonstrated that the Wilms tumor 1 (WT1) gene as detected in mRNA of endothelium is expressed in the endothelium of hemangiomas but not in vascular malformations. Leon-Villapalos et al. also confirmed that the erythrocyte-type glucose transporter protein GLUT-1 is usually expressed in hemangiomas but not vascular malformations. Serum and urine markers are less helpful in differentiating between the two anomalies.
The etiology of vascular malformation development is incompletely understood but molecular studies suggest abnormalities in signaling processes that regulate cellular proliferation and apoptosis, differentiation, maturation, and adhesion are possible causes. While several families demonstrating autosomal dominant inheritance of vascular malformations have been identified and are being studied, the overwhelming majority of vascular malformations occur sporadically and lack any Mendelian pattern of inheritance. Vascular malformations are known components of several syndromes involving the maxillofacial region. The most germane are mentioned in the following sections of this chapter. It is interesting to note that a recent study found that individuals with Down syndrome have an 80% risk reduction of vascular anomalies.
Subclassification of Vascular Malformations
Vascular malformations are described and classified by the vessels involved and their flow characteristics. Anatomically, malformations may be composed of capillaries, lymphatic vessels, veins, arteries, or a combination of the above and are named accordingly (e.g., capillary malformation, lymphatic malformation, venous-lymphatic malformation, arterio-venous malformation, etc.). Dynamically, malformations may demonstrate slow or fast fluid flow. Slow-flow malformations include capillary, lymphatic, and venous malformations alone or in any combination. Fast-flow malformations are any that contain an arterial component (e.g., arterial or arteriovenous malformations).
Understanding the nature and behavior of vascular malformations based on the Mulliken and Glowacki classification has allowed surgeons to develop rational treatment strategies that have improved safety and care of patients with these difficult lesions. Evaluation and treatment of each type of malformation is detailed later. It should be noted that combination vascular malformations are treated based on the characteristics of the predominant, deeper malformation.
Subclassification of Vascular Malformations
Vascular malformations are described and classified by the vessels involved and their flow characteristics. Anatomically, malformations may be composed of capillaries, lymphatic vessels, veins, arteries, or a combination of the above and are named accordingly (e.g., capillary malformation, lymphatic malformation, venous-lymphatic malformation, arterio-venous malformation, etc.). Dynamically, malformations may demonstrate slow or fast fluid flow. Slow-flow malformations include capillary, lymphatic, and venous malformations alone or in any combination. Fast-flow malformations are any that contain an arterial component (e.g., arterial or arteriovenous malformations).
Understanding the nature and behavior of vascular malformations based on the Mulliken and Glowacki classification has allowed surgeons to develop rational treatment strategies that have improved safety and care of patients with these difficult lesions. Evaluation and treatment of each type of malformation is detailed later. It should be noted that combination vascular malformations are treated based on the characteristics of the predominant, deeper malformation.
Slow-flow Malformations: Capillary, lymphatic, venous
Slow-flow malformations are listed in ascending order of frequency and flow characteristics:
Capillary Malformations
Capillary malformations (CMs) consist of postcapillary venules that have a decreased density of precapillary neurons, leading to the theory that diminished neural modulation of vascular tone may play a role in their development. They have frequently been referred to as “port-wine stains” and have a birth prevalence of 0.3% with equal sex distribution. They often occur in dermatomal distributions. On the face (a common location for CMs), 45% of lesions are restricted to one of the three trigeminal dermatomes. The remaining 55% of facial CMs may occur in overlapping ipsilateral dermatomes or they may cross the facial midline in a bilateral distribution. CMs are usually evident at birth and are often confused with the common vascular birthmark termed the nevus flammeus neonatorum. Nevus flammeus occurs in 50% of white newborns and is named according to its location (“angel kiss” when on the upper face and “stork bite” when on the nuchal area). These vascular birthmarks have an unknown etiology, are harmless, and typically disappear within a year. If they persist beyond a year, then reevaluation of the presumed diagnosis is required.
CMs occur sporadically with rare familial cohorts demonstrating an inheritance pattern and are associated with several syndromes. Sturge-Weber is the most common syndrome associated with CMs. The ophthalmic (V1) dermatome of the trigeminal nerve is always involved with possible additional involvement of the maxillary (V2) and mandibular (V3) distributions and ipsilateral ocular and leptomeningeal vascular anomalies. If extensive, intracranial vascular anomalies can cause refractory seizures, contralateral hemiplegia, and delayed motor and cognitive development.
Clinical Features
CMs present at birth as macular, pink/red cutaneous stains that blanch with pressure. Unlike CMs of the trunks and limbs, facial CMs typically develop a deeper hue and are prone to nodular fibrovascular overgrowth in adulthood. It has been noted that patients with facial CMs do not develop acne in the area of the malformation. There is often enlargement of the soft tissue and underlying skeleton, most notably in the maxillofacial region. In the oral and maxillofacial surgery literature, these malformations are often erroneously reported as “hemangiomas.” When an underlying skeletal deformity caused by a CM is treated by orthognathic surgery, the case is then reported incorrectly as orthognathic surgery in the setting of a “hemangioma” of the jaw(s).
Diagnostic Studies
No specific laboratory tests are required for CMs. Radiological studies such as MRI or Doppler ultrasound are performed to rule out other associated vascular anomalies that may be deep to the superficial capillary malformation.
Treatment
CMs are treated for esthetic purposes or to prevent overgrowth of adjacent tissue such as the maxillofacial skeleton. Prior to the advent of lasers, camouflage with flesh color tattooing or coagulation by electrocauterization was commonly used to manage CMs. Pulsed dye lasers, which have organic molecules pumped by an argon laser, are now considered the treatment of choice. They work best on lateral facial CMs in persons with fair skin who tan poorly (Fitzpatrick types I and II). Using wavelengths of 577 nm to 585 nm with a spot size of 5 to 10 mm and energy of 6 to 8 joules/cm 2 at short pulse durations (0.45 to 3 milliseconds) allows selective thermolysis by targeting hemoglobin (the chromophore) to a depth no more than 1.2 mm. This produces little damage to surrounding tissue. The skin is generally cooled with cool air, ice, or 10 to 50 millisecond spurts of cryogen immediately prior to laser exposure. This reduces epidermal melanin absorption. Ten percent overlap of treatment areas ensures the best result.
There is controversy with regard to treatment timing, with some advocating treatment in infancy and others believing age makes no difference. If not anesthetized, patients can expect to feel a “snap” and warm sensation at the treatment area followed by an itch that can last up to 30 minutes. Multiple treatments are necessary, especially in older patients with more mature CMs. Successful lightening occurs in up to 80% of lesions.
The small fibrovascular nodules that can occur are excised, and contour resections of adjacent soft tissue hypertrophy as well as osseous overgrowths can also be completed. Skeletal overgrowth resulting in malocclusion is treated with presurgical orthodontics and orthognathic surgery. The latter is not contraindicated in the setting of pure CMs and is usually performed using hypotensive anesthesia. Mucosal incisions for orthognathic surgery in the presence of CMs can be expected to bleed more profusely than usual but can be easily managed. Dental extractions may also be performed with use of local hemostatic measures.
Posttreatment Care
After laser treatment, the area undergoes stages of color change: initially, a blue-gray discoloration, followed by darkening to almost black over the next 24 hours. This lasts between 10 to 14 days, after which the treated area will appear red. Fading or lightening of the CM becomes evident during the next 2 to 4 weeks (about a month after treatment). Patients will have postoperative discomfort akin to sunburn for which analgesics are prescribed. Ice should be applied to the treated areas for the first 24 hours to reduce swelling. Both hyperpigmentation and hypopigmentation can occur after treatment, though hyperpigmentation generally fades in 8 to 12 weeks. Aloe or Aquaphor (Beiersdorf AG, Hamburg, Germany), can be applied daily until the skin lightens. Hypopigmentation is usually temporary. All patients should be reminded to wear sunscreen throughout the year, and in up to 50% of patients the lesion darkens again in 3 to 4 years.
Lymphatic Malformations
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


