3Etiology and Risk Factors: Biological Complications
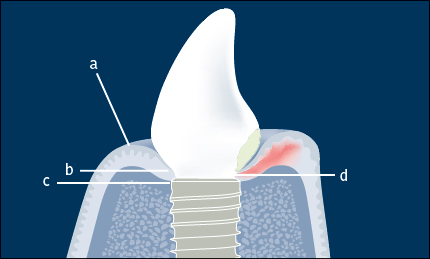
In health, the peri-implant soft tissue forms a collar around the implant/abutment (or implant/prosthesis) junction. The peri-implant soft tissue consists of an epithelial component (keratinized oral epithelium and non-keratinized junctional epithelium) and a connective-tissue component (Fig 1). The epithelium is separated from the peri-implant marginal bone by approximately 1–1.5 mm of connective tissue. The coronoapical dimension of the junctional epithelium is approximately 2 mm; however, this may vary depending on factors including the depth of implant placement, the soft-tissue phenotype, and the type of implant-abutment connection. When the peri-implant mucosa is healthy there are no clinical signs of inflammation (i.e. no bleeding on gentle probing).
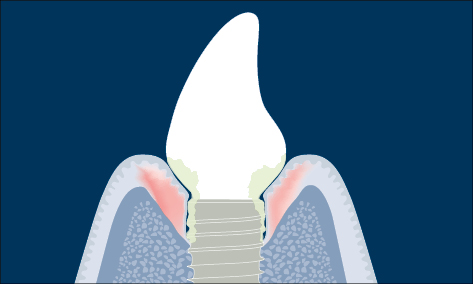
Fig 2 Schematic diagram of peri-implantitis (marginal bone loss and clinical signs of inflammation).
Once exposed to the oral cavity, microorganisms rapidly colonize the transmucosal part of the implant/abutment. In health, there is equilibrium between the bacterial challenge and the host response. The peri-implant soft tissue may be considered as a barrier that protects the zone of osseointegration from factors released from bacterial plaque and the oral cavity.
Peri-implant infections (also referred to as peri-implant diseases) are caused when an imbalance between the bacterial biofilm challenge and the host defense occurs, resulting in an inflammatory process.
When there are clinical signs of inflammation of the peri-implant mucosa (bleeding on gentle probing, 0.25 N) without the loss of supporting bone, the definition is peri-implant mucositis (Zitzmann and Berglundh 2008) (Fig 1).
When there are clinical signs of inflammation (bleeding on gentle probing, 0.25 N) in addition to loss of supporting bone the definition is peri-implantitis (Zitzmann and Berglundh 2008) (Fig 2).
In the case of peri-implantitis, probing depths greater than 5 mm with suppuration and/or bleeding on probing are frequently present (Figs 3a–b).
Recession of the peri-implant mucosa may also be associated with peri-implant infections (Fig 4).
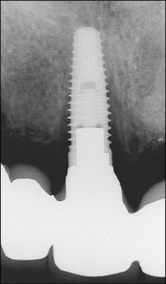
Fig 3b Periapical radiograph showing peri-implant bone loss associated with the same implant as in Fig 3a with the prosthesis in place. The marginal bone is approximately at the level of the eighth thread.
The cause-and-effect relationship between biofilm formation at implants and peri-implant mucositis has been clearly demonstrated in humans (Pontoriero and coworkers 1994; Zitzmann and coworkers 2001; Salvi and coworkers 2012). In these studies, when oral hygiene was discontinued in order to allow undisturbed plaque accumulation, clinical signs of peri-implant inflammation (bleeding on probing) appeared after a few days and resolved when oral hygiene was reinstated (Salvi and coworkers 2012).
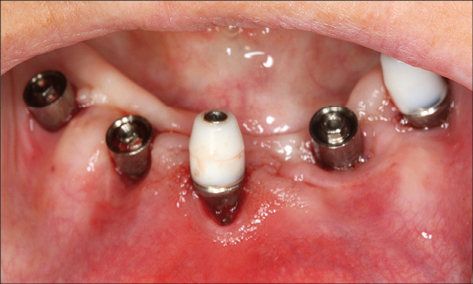
The composition of peri-implant biofilms associated with this inflammation, which may lead to further peri-implant infection in a susceptible host, is influenced by the local environment and is very similar to the microbiota on the remaining teeth in partially dentate subjects. Therefore, subjects with untreated periodontal disease, residual deep periodontal pockets, and/or poor oral hygiene are at greater risk for the development of peri-implantitis (Costa and coworkers 2012; Heitz-Mayfield and Huynh-Ba 2009; Ferreira and coworkers 2006) (Figs 5 and 6).
Biofilms associated with peri-implant infection (peri-implant mucositis and peri-implantitis) have been studied extensively using various microbiological techniques. The majority of studies has found the composition of the submucosal microbiota to be similar to that found in chronic periodontitis, with a mixed anaerobic infection dominated by Gram-negative bacteria. Some studies, however, have found high numbers of other microorganisms not commonly associated with periodontal disease, including enteric rods and yeasts, or microorganisms associated with extraoral infections such as staphylococci (Staphylococcus aureus and Staphylococcus epidermidis) or peptostreptococci (Leonhardt and coworkers 2003; Fürst and coworkers 2007; Persson and coworkers 2010). The microbiota associated with peri-implant mucositis appear to be similar to those associated with peri-implantitis (Máximo and coworkers 2009; Casado and coworkers 2011), suggesting that supramucosal plaque formation and the development of peri-implant mucositis are the precursors to peri-implantitis.
3.1.2Risk Factors for Peri-implant Infection
Inadequate plaque control
Poor oral hygiene or any factor that prevents adequate plaque control around implants can be considered a risk for peri-implant infection (Ferreira and coworkers 2006).
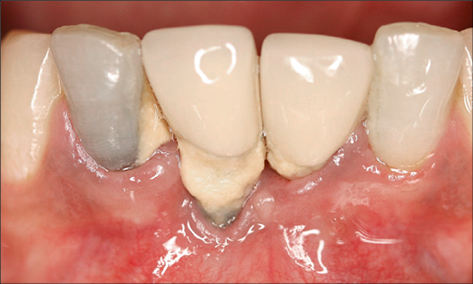
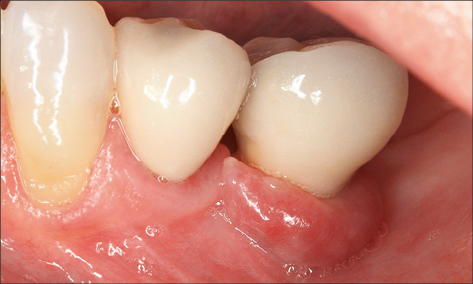
Inadequate width of keratinized peri-implant mucosa
An inadequate width of attached keratinized peri-implant mucosa has been found to be associated with an increased risk of peri-implant infection in some studies (Schrott and coworkers 2009; Lin and coworkers 2013; Brito and coworkers 2014). It is suggested that the lack of keratinized mucosa may compromise the ability to maintain an adequate plaque control. Furthermore, impaction of food particles or foreign bodies may occur, causing an infection (Figs 7 and 8).
Iatrogenic risk factors
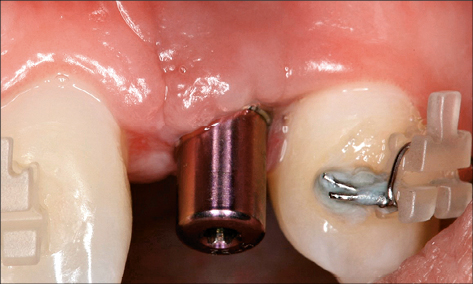
Surgical phase. Surgical-phase iatrogenic risk factors associated with peri-implant infection include malpositioned implants and inadequate grafting techniques. Implants placed too close together, or too close to neighboring teeth, do not allow adequate space for oral hygiene procedures and plaque control (Fig 9) (Abi Nader and coworkers 2014).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses