Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Understand the microbial etiology of apical periodontitis.
- 2.
Describe the routes of entry of microorganisms to the pulp and periradicular tissues.
- 3.
Recognize the different types of endodontic infections and the main microbial species involved in each one.
- 4.
Understand the bacterial diversity within infected root canals.
- 5.
Describe the factors involved with symptomatic endodontic infections.
- 6.
Understand the ecology of the endodontic microbiota and the features of the endodontic ecosystem.
- 7.
Discuss the role of microorganisms in the outcome of endodontic treatment.
- 8.
Understand the development and implications of extraradicular infections.
Learning Objectives
After reading this chapter, the student should be able to:
- 1.
Understand the microbial etiology of apical periodontitis.
- 2.
Describe the routes of entry of microorganisms to the pulp and periradicular tissues.
- 3.
Recognize the different types of endodontic infections and the main microbial species involved in each one.
- 4.
Understand the bacterial diversity within infected root canals.
- 5.
Describe the factors involved with symptomatic endodontic infections.
- 6.
Understand the ecology of the endodontic microbiota and the features of the endodontic ecosystem.
- 7.
Discuss the role of microorganisms in the outcome of endodontic treatment.
- 8.
Understand the development and implications of extraradicular infections.
Microbial Causation of Apical Periodontitis
Apical periodontitis is an inflammatory disease of microbial etiology primarily caused by infection of the root canal system ( Fig. 3.1 ). The unequivocal role of microorganisms in the causation of apical periodontitis was established nearly 40 years ago, and a huge amount of new information about the microbiology of endodontic infections has emerged in the past decade. Endodontic infections usually develop after pulpal necrosis or in cases in which the pulp was removed for treatment. Although fungi, viruses and, more recently, Archaea have been found in endodontic infections, bacteria are the major microorganisms implicated in the etiology of apical periodontitis. Bacteria colonizing the root canal system contact the periradicular tissues via apical and lateral foramina. As a consequence of the encounter between bacteria and host defenses, inflammatory changes take place in the periradicular tissues and give rise to the development of apical periodontitis. Following pulp necrosis, bacteria can grow uninhibited by host defense mechanisms. They form polymicrobial biofilms that invade all complex anatomy of the root canal system and irritate the periradicular tissues.
The ultimate goal of endodontic treatment is either to prevent the development of apical periodontitis or to create adequate conditions for periradicular tissue healing. Taking into account the microbial etiology of apical periodontitis, the rationale for endodontic treatment is unarguably to eradicate the occurring infection or to prevent microorganisms from infecting or reinfecting the root canal or the periradicular tissues. The purpose of this chapter is to describe the microbiologic aspects of endodontic infections.
Routes of Root Canal Infection
Under normal conditions, the dental pulp and dentin are sterile and isolated from oral microorganisms by overlying enamel and cementum. There are situations in which the integrity of these protective layers is breached (e.g., as a result of caries, trauma-induced fractures and cracks, restorative procedures, scaling and root planing, attrition, or abrasion) or naturally absent (e.g., because of gaps in the cementoenamel junction at the cervical root surface). Occasionally, congenital anomalies of teeth, such as dens invaginatus, dens evaginatus, or palatal groove defects, result in spontaneous pulp exposures. As a consequence, the dentin-pulp complex is exposed to the oral environment and put at risk of infection by oral microorganisms. Microorganisms from subgingival biofilms associated with periodontal health or disease may also reach the pulp via dentinal tubules, or lateral and apical foramina, or possibly as a result of a systemic route.
Dentinal Tubules
Whenever dentin is exposed, the pulp is put at risk for infection as a consequence of the permeability of normal dentin, which is dictated by its tubular structure. Dentin permeability is increased near the pulp because of the larger diameter and higher density of tubules. Exposed dentin can be challenged by microorganisms present in carious lesions, in saliva bathing the exposed area, or in dental plaque formed onto the exposed area.
Dentinal tubules traverse the entire width of the dentin and have a conformation of inverted cones, with the smallest diameter in the periphery, near enamel or cementum (mean of 0.9 µm). The smallest tubule diameter is entirely compatible with the cell diameter of most oral bacterial species, which usually ranges from 0.2 to 0.7 µm. Bacterial invasion of dentinal tubules occurs more rapidly in nonvital teeth than in vital ones. In vital teeth, outward movement of dentinal fluid and the tubular contents influence dentinal permeability and can conceivably delay intratubular invasion by bacteria. Other factors, such as dentinal sclerosis beneath a carious lesion, reparative or reactionary dentin, smear layer, and intratubular accumulation of host defense molecules, such as antibodies, also limit or even impede bacterial progression to the pulp via dentinal tubules. Thus, as long as the pulp is vital, dentinal exposure does not represent a significant route of pulpal infection, except when dentin thickness is considerably reduced; then, dentin permeability is significantly increased. On the other hand, if the pulp is necrotic, exposed dentinal tubules can become true avenues for bacteria to reach and colonize the pulp.
Direct Pulp Exposure
Direct exposure of the dental pulp to the oral cavity is the most obvious route of endodontic infection. Caries is the most common cause of pulpal exposure, but microorganisms may also reach the pulp via direct pulpal exposure as a result of iatrogenic restorative procedures or trauma. There is a distinct difference, though, between the two processes of pulp exposure. Caries is a chronic disease that takes months to years to reach an exposure. Therefore, the pulp in the case of the carious exposure is exposed to biofilms containing high bacterial loads for a long period. The pulp in these cases is significantly inflamed and occasionally has an abscess formation at the site of the exposure, even though the patient may be asymptomatic or mildly symptomatic. With mechanical exposure, however, only a few planktonic bacterial cells gain access to the pulp, particularly if a rubber dam is in place during preparation.
The reaction of the pulp, therefore, to the two types of exposure is widely different (see Chapter 2 ). The exposed pulp tissue develops direct contact with oral microorganisms from carious lesions, saliva, or plaque accumulated onto the exposed surface ( Fig. 3.2 ). Almost invariably, exposed pulps undergo inflammation and necrosis and become infected. The time lapse between pulp exposure and infection of the entire canal is unpredictable, but it is usually a slow process.

Periodontal Disease
In either normal or diseased periodontal tissues, microorganisms in subgingival biofilms could reach the pulp through dentinal tubules or lateral/furcal canals. As noted, the outward flow of dentinal fluid is protective of the pulp. However, pulpal necrosis as a consequence of periodontal disease develops only if the periodontal pocket reaches the apical foramen, leading to irreversible damage to the main blood vessels that penetrate through this foramen. Once the pulp becomes necrotic, periodontal microorganisms can reach the root canal system via ramifications, exposed dentinal tubules, and apical foramina and establish an infectious process (see Chapter 7 ).
Anachoresis
Anachoresis is a process by which microorganisms are transported in the blood or lymph to an area of tissue damage, where they leave the vessel, enter the damaged tissue, and establish an infection. There is no clear evidence that this process represents a route for root canal infection. Research has shown that bacteria could not be recovered from unfilled root canals, when the bloodstream was experimentally infected, unless the root canals were overinstrumented during the period of bacteremia, with resulting injury to periodontal blood vessels and blood seepage into the canal. Current evidence indicates that the main pathway of pulpal infection in traumatic injuries is from the gingival sulcus, through dentinal exposure as a result of enamel cracks or the microvasculature of the traumatized periodontal ligament.
Microbiota of Endodontic Infections
Endodontic infections can be classified according to the anatomic location as intraradicular or extraradicular. Intraradicular infections can in turn be subdivided into three categories: primary, secondary, or persistent infection, depending on when the participating microorganisms established themselves in the root canal.
The composition of the microbiota may vary, depending on the different types of infection and different forms of apical periodontitis. Studies using culture-dependent approaches have allowed recognition of several candidate endodontic pathogens. More recently, with the advent of culture-independent molecular biology techniques, not only have the findings from culture studies been confirmed, but also a great deal of new information has been added to our knowledge of the microbiota associated with different types of endodontic infections. Molecular technology has enabled the recognition of new putative pathogens that had never been found in endodontic infections. Moreover, many species that had already been considered putative pathogens because of their frequency of detection, as reported by culture-dependent methods, have been found in a similar or even higher prevalence by molecular approaches, strengthening these organisms’ association with causation of apical periodontitis. As a consequence, the endodontic microbiota has been clearly redefined by molecular biology methods. The next sections discuss the main characteristics of the different types of endodontic infections.
Primary Intraradicular Infection
Microorganisms that initially invade and colonize the necrotic pulp tissue cause primary intraradicular infection. Participating microorganisms can be involved in the earlier stages of pulpal invasion, which culminate in inflammation and further necrosis, or they can be latecomers that take advantage of the environmental conditions in the canal after pulp necrosis. Primary infections are characterized by a mixed consortium composed of 10 to 30 bacterial species and 10 3 to 10 8 bacterial cells per canal.
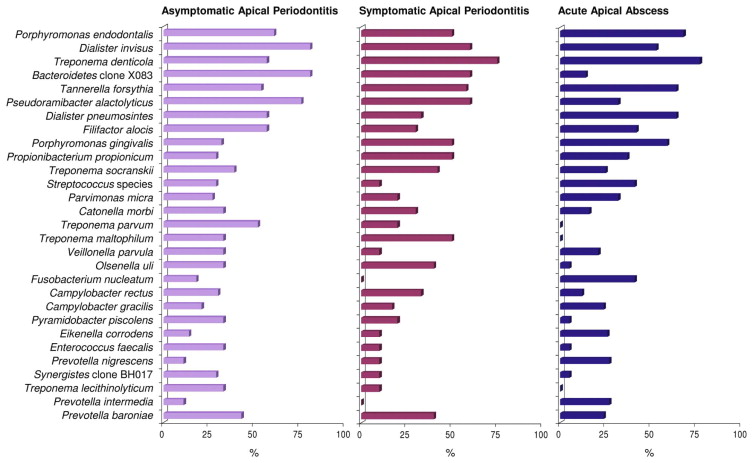
Sophisticated culture and molecular biology techniques have revealed the polymicrobial nature of endodontic infections, with a conspicuous dominance of obligate anaerobic bacterial species in primary infections. At a broader taxonomic level, endodontic bacteria fall into nine phyla, namely, Firmicutes, Bacteroidetes, Spirochaetes, Fusobacteria, Actinobacteria, Proteobacteria, Synergistetes , TM7, and SR1. However, data from studies using advanced DNA sequencing technologies reveal that several other phyla may have been overlooked by previous identification techniques (see the following sections). Noteworthy is the high prevalence of as-yet-uncultivated species; about 40% to 66% of the endodontic microbiota is composed of bacteria that have yet to be cultivated and fully characterized. In addition, bacterial profiles of the endodontic microbiota vary from individual to individual, suggesting that apical periodontitis has a heterogeneous etiology in which multiple bacterial combinations can play a role in disease causation. Table 3.1 shows the bacterial genera commonly found in endodontic infections, and Fig. 3.3 shows the most prevalent bacterial taxa found in primary intraradicular infections associated with different forms of apical periodontitis.
| Gram-negative bacteria | Gram-positive bacteria | ||
|---|---|---|---|
| Anaerobes | Facultatives | Anaerobes | Facultatives |
| Rods | Rods | ||
|
|
|
|
| Cocci | Cocci | ||
|
|
|
|
| Spirilla | |||
|
|||
Gram-Negative Bacteria
Gram-negative bacteria appear to be the most common microorganisms in primary endodontic infections. Species belonging to several genera of gram-negative bacteria have been consistently found in primary infections associated with different forms of apical periodontitis, including abscesses. These genera include Dialister (e.g., D. invisus and D. pneumosintes ), Fusobacterium (e.g., F. nucleatum ), Porphyromonas (e.g., P. endodontalis and P. gingivalis ), Prevotella (e.g., P. intermedia , P. nigrescens, P. baroniae and P. tannerae ), Tannerella (e.g., T. forsythia ), and Treponema (e.g., T. denticola and T. socranskii ). Other gram-negative bacteria detected more sporadically in primary infections are shown in Table 3.1 .
Gram-Positive Bacteria
Even though anaerobic gram-negative bacteria are reported to be the most common microorganisms in primary infections, several gram-positive bacteria have also been frequently detected in the endodontic mixed consortium, some of them in prevalence values as high as the most commonly found gram-negative species. The genera of gram-positive bacteria often found in primary infections include Actinomyces (e.g., A. israelii ), Filifactor (e.g., F. alocis ), Olsenella (e.g., O. uli ), Parvimonas (e.g., P. micra ), Peptostreptococcus (e.g., P. anaerobius, P. stomatis ), Pseudoramibacter (e.g., P. alactolyticus ), Streptococcus (e.g., S. anginosus group), and Propionibacterium (e.g., P. propionicum and P. acnes ). Other gram-positive bacteria found more sporadically in primary intraradicular infections are listed in Table 3.1 .
As-Yet-Uncultivated Bacterial Phylotypes
Data from culture-independent molecular biology studies have indicated that several bacterial phylotypes can participate in endodontic infections. Phylotypes can be regarded as species that have not yet been cultivated and validly named and are known only by a 16S rRNA gene sequence. Uncultivated phylotypes have been frequently detected in samples from primary endodontic infections, including phylotypes belonging to the genera Dialister, Prevotella , Solobacterium, Olsenella, Eubacterium , and Megasphaera; the Lachnospiraceae family; and the Synergistetes phylum. These phylotypes are previously unrecognized bacteria that may play a role in the pathogenesis of apical periodontitis. The fact that they have yet to be cultivated and phenotypically characterized does not mean that they are not important.
Complexity of Endodontic Polymicrobial Infections
For decades, the identification of endodontic microorganisms was performed using culturing techniques. There are advantages to being able to culture an organism, such as learning its growth requirements, virulence factors, and antibiotic sensitivity; this knowledge allows researchers to develop antimicrobial strategies. However, it appears that only about 10% of the human microbiome (i.e., the microbial communities that populate the human body) is cultivable. Even with the advent of molecular techniques, studies sought first to identify known, cultivable bacteria with higher sensitivity than culturing and then to use techniques that relied on the cloning and sequencing of a few organisms per specimen. The latter techniques helped identify many organisms that have never been cultivated. The technology of molecular sequencing has advanced so rapidly that a massive amount of sequencing per specimen now is possible and affordable. These technologies, which are referred to as “next-generation sequencing,” or pyrosequencing, have allowed much greater depth of coverage in the identification of endodontic microorganisms. Studies using pyrosequencing have revealed that endodontic infections may contain bacteria from 10 to 24 different phyla, with hundreds of taxa identified. In addition, these studies can provide considerable information about the true differences in microbial diversity between acute and chronic infections and about the transition of the microbiota from a healthy oral condition to an endodontic infection.
Other Microorganisms in Endodontic Infections
Microorganisms other than bacteria occasionally have been found in endodontic infections. Fungi are eukaryotic microorganisms that have been only sporadically found in primary infections. Archaea comprise a highly diverse group of prokaryotes, distinct from bacteria, with no known human pathogen. One study found methanogenic Archaea in 25% of the canals of teeth with chronic apical periodontitis, but this relatively high prevalence was not confirmed by other studies. Viruses are not cells but inanimate particles that have no metabolism on their own. Because viruses require viable host cells to infect and replicate themselves, they cannot survive in the root canal with necrotic pulp. Viruses have been reported to occur in the root canal only in teeth with vital pulps. For instance, the human immunodeficiency virus (HIV) has been detected in noninflamed vital pulps of patients who are HIV seropositive, and some herpes viruses have been identified in both noninflamed and inflamed vital pulps. Several herpes viruses have been found in inflamed periradicular tissues, including symptomatic apical periodontitis lesions and abscesses. The specific role of viruses in the pathogenesis of pulpitis and apical periodontitis, if any, remains to be elucidated.
Symptomatic Infections
It has been suggested that the probability of symptoms is increased when certain bacterial species are part of the infective endodontic microbiota. Nevertheless, the same species can be found in asymptomatic cases with a prevalence comparable to that of symptomatic cases. This raises the suspicion that factors other than the mere presence of a given putative pathogenic species can influence the development of symptoms. These factors include differences in virulence ability among strains of the same species; the number of occurring species and interactions among them that result in additive or synergistic pathogenic effects; the number of bacterial cells (load); the environmental cues regulating expression of virulence factors; host resistance; and concomitant herpes virus infection. Association of some or all of these factors (rather than an isolated event) is likely to dictate the occurrence and intensity of symptoms.
Ecology of the Endodontic Microbiota
A root canal with necrotic pulp provides a space for bacterial colonization. It also gives bacteria a moist, warm, nutritious, and anaerobic environment that is mostly protected from host defenses because of the lack of active microcirculation in the necrotic tissue. Intuitively, the necrotic root canal might be considered a rather fertile environment for bacterial growth, and it might be realized that colonization should not be a difficult task for virtually every oral bacterial species. Although approximately 1,000 different bacterial taxa have been reported in the oral cavity, and each individual’s mouth can harbor about 100 to 200 taxa, only a restricted assortment of these bacteria is found in an infected canal. This indicates that selective pressures must occur in the root canal system that favor the establishment of some species and inhibit others. The key ecologic factors that influence the composition of the microbiota in the necrotic root canal include oxygen tension and redox potential, the type and amount of available nutrients, and bacterial interactions.
Oxygen Tension and Redox Potential
Root canal infection is a dynamic process, and different bacterial species apparently dominate at different stages of the infectious process. Early in the initial phases of the pulpal infectious process, facultative bacteria predominate. After a few days or weeks, oxygen is depleted in the root canal as a result of pulp necrosis and consumption by facultative bacteria. An anaerobic milieu develops, with consequent low redox potential; this is highly conducive to the survival and growth of obligate anaerobic bacteria. With the passage of time, anaerobic conditions become even more pronounced, particularly in the apical third of the root canal, and as a consequence, anaerobes come to dominate the microbiota and outnumber facultative bacteria.
Available Nutrients
In the root canal system, bacteria can utilize the following as sources of nutrients: (1) the necrotic pulp tissue, (2) proteins and glycoproteins from tissue fluids and exudate that seep into the root canal system via apical and lateral foramina, (3) components of saliva that may coronally penetrate into the root canal, and (4) products of the metabolism of other bacteria. Because the largest amount of nutrients is available in the main canal, which is the most voluminous part of the root canal system, most of the infecting microbiota, particularly fastidious anaerobic species, is expected to be located in this region. At later stages of the infectious process, nutritional conditions favor the establishment of bacteria that metabolize peptides and amino acids.
Bacterial Interactions
The establishment of certain species in the root canal is also influenced by interactions with other species. Positive interactions (mutualism and commensalism) enhance the survival capacity of the interacting bacteria and increase the probability of certain species coexisting in the habitat. Negative interactions (competition and antagonism) limit population densities.
Apical Periodontitis as a Biofilm-Related Disease
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


