Introduction
Effects of sodium fluoride (NaF) mouth rinse solutions on torsional properties of a miniscrew implant were investigated.
Methods
As-received Ti-6Al-4V miniscrew implants (AbsoAnchor [Dentos, Inc., Daigu, Korea]) were immersed in 0.1% or 0.2% NaF mouth rinse solution (pH 5.12 and 5.14, respectively) for 1 hour or 24 hours. Miniscrew implants selected as controls were not immersed. Each implant was subjected to increasing torque until fracture (n = 5 in sample groups). Mean moment and twist angle for fracture were compared using 1-way analysis of variance (ANOVA). Surfaces of implants after immersion were observed with a scanning electron microscope (SEM). Electron microprobe and micro-x-ray diffraction analyses were performed to obtain composition information about deposits on implant surfaces.
Results
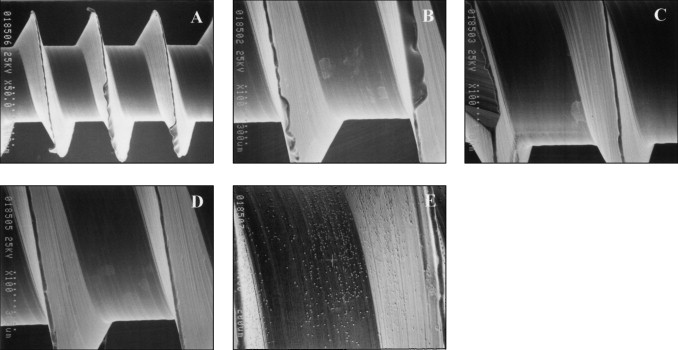
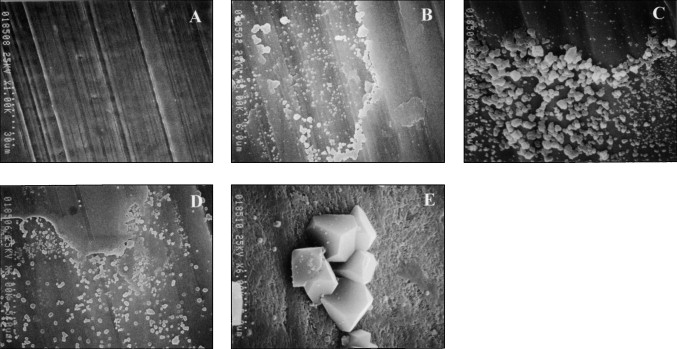
Pits and cracks formed on the implant surfaces after immersion in both NaF mouth rinse solutions. Corrosion products, probably sodium aluminum fluoride (Na 3 AlF 6 ), were observed on the implants after immersion in both NaF solutions for both time periods. There were no significant differences for mean torque ( P = 0.063) and twist angle ( P = 0.696) at fracture compared with control implants.
Conclusions
Although titanium alloy miniscrew implants corroded slightly from immersion in 0.1% or 0.2% NaF solutions, mouth rinsing by patients with the same fluoride solution concentrations should not cause deterioration of their torsional performance.
Since the technique employing implants for skeletal anchorage was introduced, titanium miniscrew implants have become very popular because of lower cost, simpler placement, and removal with less traumatic surgery and less discomfort for patients. However, we have experienced occasional fracture of the miniscrew implants during placement or removal. Recent clinical studies have also reported fracture of miniscrew implants. Miniscrew implant fracture is a serious problem for the orthodontist and patient because it is sometimes difficult to remove the implant fragment from inside the bone.
Because the demineralization of enamel adjacent to an orthodontic fixed appliance is a significant clinical problem, fluoride-containing products such as toothpaste, bonding materials, and solutions for prevention of dental caries are commonly used in clinical orthodontics. Mouth rinsing with fluoride-containing products is an effective method for prevention of caries because orthodontic fixed appliances have complicated morphologies. However, reduced corrosion resistance of pure titanium and titanium alloys in fluoride-containing environments that attack the protective surface oxide has been reported, because F − ions in the solution combine with H + ions to form hydrogen fluoride (HF), even if the NaF concentration is low. Clinically available fluoride-containing products have a variety of fluoride concentrations (250 to 10,000 ppm) and pH (approximately 3.5 to 7.0). The corrosion of titanium is affected not only by fluoride concentration but also by pH. Therefore, titanium corrodes in the presence of a small amount of NaF if the value of pH is sufficiently low, and even corrodes at high pH if the NaF concentration is considerably high. Although fluoride solutions should be important to prevent dental caries for orthodontic patients, use of these solutions may cause fracture of the miniscrew implants because the commercial products are manufactured from pure titanium and titanium alloys.
No previous studies have been performed to study the effect of immersion in fluoride solutions on the torsional properties of miniscrew implants. The purpose of this study was to investigate the effects of immersion in NaF mouth rinse solutions on the torsional properties of 1 miniscrew implant product.
Material and methods
Materials
As-received titanium alloy (Ti-6Al-4V) miniscrew implants with 1.6-mm diameter and 12.0-mm length were used (AbsoAnchor SH-1615-12, Dentos, Daegu, Korea). Miniscrew implants were ultrasonically cleaned with ethanol and 2-propanone. Some miniscrew implants were immersed in 0.1% (450 ppm fluoride [F]) or 0.2% (900 ppm F) NaF mouth rinse solution (Ora-Bliss, Showa Yakuhin Kako Ltd, Tokyo, Japan) for 1 hour or 24 hours. Other implants selected as controls were not immersed. The pH values were 5.12 for the 0.1% NaF solution and 5.14 for the 0.2% NaF solution.
Torsional test
After immersion in 1 of the 2 NaF solutions for 1 of the 2 time periods, each miniscrew implant was subjected to increasing torsional moment, using a custom-fabricated device, until fracture occurred. A rotational speed of 90° per minute was employed, and values of torsional moment were originally obtained in units of kilogram force (kf) × centimeters and then converted to units of newtons (N) × centimeters.
Scanning electron microscope observations
The surfaces of the miniscrew implants before (control) and after immersion were observed with a scanning electron microscope (SEM) (X-650, Hitachi, Tokyo, Japan). One as-received miniscrew implant was encapsulated in epoxy resin (Epofix, Struers, Copenhagen, Denmark) for electron microprobe analysis (EMPA). The surface of this sample implant was ground and polished using a series of silicon carbide abrasive papers and a final slurry of 0.05-μm alumina particles. After polishing, this mounted sample implant was ultrasonically cleaned with ethanol and 2-propanone and immersed in the 0.2% NaF mouth rinse solution for 24 hours. Composition of surface regions was subsequently determined by EMPA.
Micro-x-ray diffraction
The miniscrew implants were analyzed before and after immersion with Micro-x-ray diffraction (Micro-XRD; Rint-2000, Rigaku Corp., Tokyo, Japan). The Micro-XRD analyses were performed with Cu Kα radiation at 40 kV and a tube current of 300 mA. The specimens were oscillated from 0° to +15° about the χ-axis and rotated from –120° to +120° about the φ-axis to minimize the effect of preferred orientation. The locations of these axes are shown in previous publications that describe the Micro-XRD technique. A 50-μm diameter collimator was used to establish the dimensions of the analysis area. The diffracted x-rays were detected by a position-sensitive proportional counter (PSPC), and the measurement time for scanning over the diffraction angle (2) range from 30° to 100° was 10 minutes.
Statistical analysis
Mean torque values and twist angles at fracture for the 5 sample groups, each containing 5 replicate specimens (n = 5) were compared by 1-way ANOVA (Version 16.0, SPSS, Chicago, IL). The purpose of these comparisons was to assess whether F concentration (0.1% or 0.2%) or immersion time (1 hour or 24 hours) for the miniscrew implants caused significant changes in torsional properties compared with control implants that had not been immersed.
Results
Mean values with standard deviations for torque and twist angle at fracture for the 5 groups of miniscrew implants are summarized in Table I . (A second decimal place from statistical computations has been retained.) While the individual torque values at fracture were very similar for each NaF concentration and period of immersion, there was greater variation in twist angle at fracture for the different specimens. There were no significant differences for mean torque values ( P = 0.063) or for twist angle values ( P = 0.696) at fracture, compared with control miniscrew implants that were not immersed in NaF solution.
| Category | Torque (N•cm) | Twist angle (°) |
|---|---|---|
| Control (Not immersed) | 15.61 ± 0.55 | 154.51 ± 14.42 |
| 0.1% NaF solution 1-hour immersion |
14.97 ± 0.71 | 153.27 ± 21.72 |
| 0.2% NaF solution 1-hour immersion |
14.51 ± 1.08 | 158.98 ± 20.65 |
| 0.1% NaF solution 24-hour immersion |
15.43 ± 0.33 | 139.81 ± 41.28 |
| 0.2% NaF solution 24-hour immersion |
14.65 ± 0.24 | 142.02 ± 17.78 |
Representative low- and high-magnification SEM images of the miniscrew implants are shown in Figures 1 and 2 . Pits and cracks were observed on the surfaces of miniscrew implants after immersion in both NaF mouth rinse solutions ( Fig 2 ). Some corrosion products were observed on the surfaces of miniscrew implants after immersion in both NaF mouth rinse solutions ( Figs 1 and 2 ).