Introduction
Our objectives were to determine the effects of simvastatin on relapse and periodontal tissue remodeling after experimental tooth movement in rats and to explore the molecule mechanism.
Methods
Thirty-two adult male Wistar rats were randomly divided into 2 groups. Bilateral mandibular first molars were moved mesially with nickel-titanium closed-coil springs in both groups. On the 21st day, the springs were removed, and dental casts were made. Animals in the experimental group began receiving simvastatin at a dose of 2.5 mg per kilogram per day for 4 weeks, and animals in the control group received 0.9% sodium chloride. The results were evaluated by model measuring and immunohistochemistry staining.
Results
Relapse distances and relapse percentages were decreased in the simvastatin group compared with the controls. Osteoprotegerin expression increased, and RANKL decreased.
Conclusions
These results indicate that simvastatin inhibits the bone-resorbing activity of osteoclasts while stimulating bone formation, probably by controlling the ratio of local osteoprotegerin to RANKL in the periodontal tissues. Therefore, it might be useful for retention.
Relapse is a major concern in orthodontics. It is a physiologic response of the supporting tissues to force application and can be attributed mainly to the occlusal stability and the increased mechanical tension exerted by the transseptal fiber system. It is believed that, during orthodontic tooth movement, stresses and strains are built up and stored in the periodontal and transseptal fiber systems. After removal of the orthodontic appliance, these stresses are released, and the teeth begin to relapse to their original positions. However, even though the transseptal fiber system is primarily responsible for the generation of forces on moved teeth, osteoclastic resorption and osteoblastic formation of surrounding alveolar bone are necessary for relapse. Because the relapse pressure persists until alveolar bone remodeling is completed, stimulation of alveolar bone production or inhibition of bone resorption after orthodontic tooth movement should effectively prevent the relapse of moved teeth.
The most common measure to overcome relapse is a retainer. Retention is a necessary procedure in orthodontics, and it is often prescribed for long periods in general, and even longer periods in adults and patients with periodontitis. Therefore, it is important to promote tooth stability at the new position, prevent relapse, and shorten the retention time for adults and patients with periodontitis. Recently, many studies have suggested that pharmacologic therapy might provide another mechanism to control tooth movement. Kim et al clarified that systemic administration of bisphosphonate in rats decreased the extent of relapse of moved molars via a mechanism involving impairment of the structure and resorptive functions of osteoclasts. Kanzaki et al reported that the osteoprotegerin (OPG) gene transfer to periodontal tissue inhibited the receptor activator of nuclear factor κB ligand (RANKL)-mediated osteoclastogenesis and significantly inhibited experimental tooth movement. However, in these studies, the control of tooth movement was considered to be due to inhibiting bone-resorbing activity of osteoclasts. In a recent study, relaxin, which is known to remodel soft tissues, was administered to dogs by gingival injection and had a significant preventive effect on relapse. Therefore, we hypothesized that stimulating bone formation might be a method to make moved tooth stable and reduce relapse.
Statins, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA)-reductase inhibitor, are widely used for lowering serum cholesterol. Several statins, such as simvastatin and lovastatin, have anabolic effects on bone metabolism in vivo. Also, statins can stimulate osteoblastic bone formation in vitro and in vivo. Many researchers are using statins in oral research. A recent study reported, that at low concentrations, simvastatin had a positive effect on the proliferation and differentiation of human periodontal ligament (PDL) cells in vitro. Furthermore, an in-vivo study showed that simvastatin had a stimulatory effect on alveolar bone formation in rats with periodontitis. Statin and collagen grafts increased new bone formation in parietal defects in rabbits. Intraperitoneally injected simvastatin promoted osteogenesis around titanium implants measured as the bone contact ratio.
So far, no study concerning the effect of simvastatin on orthodontic tooth movement has been done. The purposes of this study were to investigate whether systemic administration of simvastatin in rats has an effect on relapse after orthodontic tooth movement and to explore the molecular mechanism of its role.
Material and methods
All procedures involving animals were in strict accordance with the guidelines established by JiLin University for animal research and approved by the local ethics committee. Thirty-two male Wistar rats (age, 7-8 weeks; weight, 225 ± 25 g) were used in this study. All rats were bred in the Jilin University Laboratory Animal Center and housed in cages under controlled conditions of temperature (22°C), humidity (40%), and lighting (12 hour light and dark cycle) with free access to food and water.
The appliance design and placement were as previously described by Leiker et al. Briefly, the animals were anesthetized with ketamine hydrochloride, and a nickel-titanium closed-coil spring exerting an orthodontic force of 50 cN was ligated bilaterally to the maxillary first molars and incisors. The force was measured with a gauge. The rationale for the applied 50 cN force was that previous studies had demonstrated that a 40 to 60 cN force stimulated substantial molar tooth movement in rats. Experimental tooth movement was conducted for 21 days. Stone models of the rat maxillae in both groups were made every week. At the end of the experimental period, the spring appliances were removed, and precise impressions of all rat maxillae were taken by using silicone material with individual resin trays with the rats under anesthesia with light ether; stone models of the maxillae were also made. The rats were then randomly divided into 2 groups: a control group and a test group receiving simvastatin injections. Rats in the test group were given intraperitoneal injections of simvastatin, 2.5 mg per kilogram per day (JingXin Pharmaceutical, ZheJiang, China), for 4 weeks from the last day of tooth movement. Rats in control group received daily injections of 0.9% sodium chloride during the same period.
To quantify tooth movement, the distance between the distal grooves of the first and second molars was measured on the stone model of each animal. The models were trimmed so that they were parallel to the occlusion plane of the molars; the distance between the bottom and the occlusal plane was about 1 cm. The prepared models were scanned with Scan Maker (version 5.99, Samsung, Beijing, China) with a 1:1 proportion, and the distance was measured bilaterally with Photoshop (version 7.0, Adobe Photoshop, San Jose, Calif). All measurements were repeated 3 times by an examiner, and the mean of these measurements was used as the representative value of each distance. The following parameters were calculated on the basis of the measurements: (1) tooth movement (D0), the total tooth movement during the 21 days of orthodontic treatment; (2) relapse distance (Ra) of the various weeks, estimated by subtracting the distances of the various weeks from D0; and (3) percentage of relapse of the various weeks (PRn), the amount of Rn times 100 divided by D0.
At the end of drug treatment, the rats in both groups were perfused transcardially with 4% paraformaldehyde in 0.1 mol/L of phosphate buffer (pH 7.4) under deep anesthesia. The maxillae, including molars, were dissected, fixed overnight in 4% paraformaldehyde buffer, and then decalcified in 10% EDTA solution (pH 7.4) at 4°C for 4 weeks. The decalcified maxillae were dehydrated in a graded series of ethanol and embedded in paraffin. Eleven consecutive cross-sections were cut at 5 μm, and the sections numbered 1, 4, 7, and 11 were selected for staining with hematoxylin and eosin and strept-avidin-biotin-horseradish peroxidase complex immunohistochemistry. The detection of OPG and RANKL immunoreactivity was performed with polyclonal rabbit anti-OPG (1:250; Santa Cruz; sc-11383) and polyclonal goat anti-RANKL (1:250; Santa Cruz, sc-7628). The immunoreactivity of OPG and RANKL proteins was scored by using the Computer Image Analyzing System (HPIAS-1000, version 6.0, Media Cybernetics, Silver Spring, Md), and then the scores were converted to gray-scale values. Periodontal tissues of the mesial roots of the first molars were examined. The expressions were measured on the pressure and tension sides in half of the mesial root area by a quantitative method, divided by the mesiodistal axis of the root.
Statistical analysis
The data were processed with SPSS software (version 11.5, SPSS, Chicago, Ill). The results were expressed as the means ± standard deviations. The data of Rn, PRn, OPG, and RANKL between groups were subjected to 1-way analysis of variance (ANOVA). The data of OPG and RANKL on the pressure and tension sides were subjected to paired t tests. The significance level was set at P <0.05.
Results
As shown in Table I , after simvastatin administration for 1 week and 4 weeks, the relapse distances in the test group were less than those in the control group ( P <0.01). PRn in the test group was significantly lower compared with the control group ( P <0.001). R1 and R4 of test group were 37.4% and 54.3% of the control group.
| Group | Relapse distance (mm) Relapse percentage (%) | |||
|---|---|---|---|---|
| 1 week | 4 weeks | 1 week | 4 weeks | |
| Control | 0.265 ± 0.034 | 0.359 ± 0.051 | 74.77 | 93.65 |
| Simvastatin | 0.099 ± 0.02 ∗ | 0.195 ± 0.032 ∗ | 26.81 ∗ | 53.38 † |
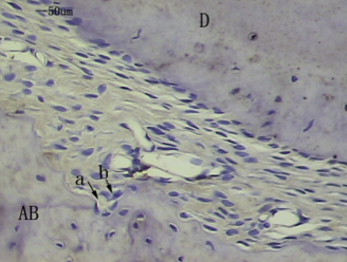
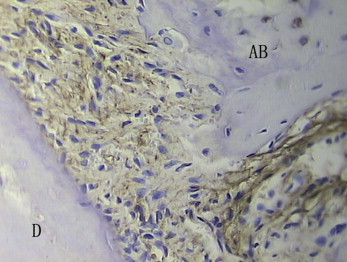
In the histologic examination, after appliance removal, the first molar relapsed toward the distal side. Therefore, the PDL on the distal side of the mesial root was considered to be the compressed side, and the mesial side of the PDL of the mesial root was the stretched side. Because tipping movement of the molars had occurred by force applications from placement and removal, we examined the PDL and the adjacent alveolar bones at the longitudinal midportion of the molar roots. In the immunohistochemistry examination, OPG was mainly distributed in fusiform mesenchymal cells around blood vessels and osteoblasts in the bone marrow, on the surface of the alveolar bone, and in fibroblasts in the periodontal tissue, odontoblasts, and dental pulp cells during normal tooth movement in the rats. RANKL was well expressed mainly in fusiform mesenchymal cells and extension cell processes around blood vessels, in addition to ostoblasts along the alveolar bone surface and a few weakly stained periodontal fibroblasts.
In the control group on the compressed side, the surfaces of alveolar bone were rough, along which there were a few monolayer ancipital osteoblasts. A few osteoclasts formed Howship’s resorption lacunae with new bone deposition. The PDLs were still irregularly arranged. OPG in the PDL was weakly and positively expressed ( Fig 1 ), but RANKL in the PDL was strongly and positively expressed ( Fig 2 ).
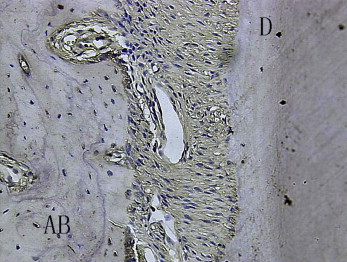
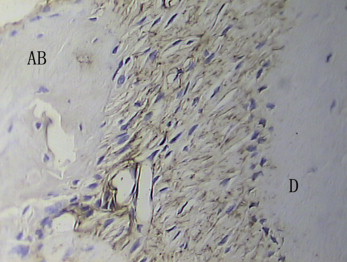
In the test group, on the compression side, bundles of dense fibers and fibroblasts were regularly distributed, and the Sharpey’s fibers were stretched into the osteoid layers. The surfaces of bone and tooth were smooth. Rows of osteoblasts were arranged on the surface of the alveolar bone, which showed a round profile and plenty of cytoplasm. The osteoid layers were thick with many deeply stained hematoxylin lines, even forming finger protrusions. There were plenty of blood vessels. OPG in the PDL was strongly positively expressed ( Fig 3 ), but RANKL in the PDL was weakly expressed ( Fig 4 ). Most of the osteoclasts were clearly localized around blood vessels in the vascular canals of the alveolar bones ( Fig 5 ) but were apparently sparse in the PDL proper on the compressed side.

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


