Introduction
A number of biologic methods leading to decreased rates of orthodontic tooth movement (OTM) can be found in the recent literature. The aim of this systematic review was to provide an overview of biologic methods and their effects on OTM inhibition.
Methods
An electronic search was performed up to January 2016. Two researchers independently selected the studies (kappa index, 0.8) using the selection criteria established in the PRISMA statement. The methodologic quality of the articles was assessed objectively according to the Methodological Index for Non-Randomized Studies scale.
Results
We retrieved 861 articles in the initial electronic search, and 57 were finally analyzed. Three biologic techniques were identified as reducing the rate of OTM: chemical methods, low-level laser therapy, and gene therapy. When the experimental objective was to slow down OTM, pharmacologic modulation was the most frequently described method (53 articles). Rats were the most frequent model (38 of 57 articles), followed by mice (9 of 57), rabbits (4 of 57), guinea pigs (2 of 57), dogs (2 of 57), cats (1 of 57), and monkeys (1 of 57). The sample sizes seldom exceeded 25 subjects per group (6 of 57 articles). The application protocols, quality, and effectiveness of the different biologic methods in reducing OTM varied widely.
Conclusions
OTM inhibition was experimentally tested with various biologic methods that were notably effective at bench scale, although their clinical applicability to humans was rarely tested further. Rigorous randomized clinical trials are therefore needed to allow the orthodontist to improve the effect of translating them from bench to clinic.
Highlights
- •
Biologic methods can inhibit the rate of orthodontic tooth movement (OTM).
- •
This systematic review examined the effects of biologic methods on OTM inhibition.
- •
Chemical methods, low-level laser therapy, and gene therapy reduce the rate of OTM.
- •
Pharmacologic modulation was the most frequently described method (53 articles).
Absolute control over tooth movement is a key factor in orthodontics. One main remaining limitation of past and current orthodontic treatments is the inability to completely prevent the unexpected movement of certain teeth; this is frequently defined as loss of anchorage during treatment or relapse during the retention phase. At present, auxiliary devices such as temporary anchorage devices are used to provide additional biomechanical resistance and help prevent undesirable tooth movement. Similarly, in recent decades, a number of biologic methods have emerged that can decrease the rate of orthodontic tooth movement (OTM) or even inhibit it completely by interfering with osteoclast cell activity during the bone remodeling on which OTM depends.
In this respect, chemical methods, including hormones, drugs, and various synthetic molecules, have been used from the earliest to the most recent studies on OTM. Bisphosphonates (inhibitors of bone resorption) and prostaglandin inhibitors, such as ibuprofen and acetylsalicylic acid, have been widely studied because of their activity in slowing OTM. Apart from the administration of specific drugs, other methods proposed in the literature to reduce the rate of OTM include processes that modify the biologic substrate, such as low-level laser therapy or gene therapy. The doses, protocols, and hypotheses are as varied as the studies themselves; this makes it difficult for the clinician to establish useful comparisons between studies and their relevance, if any, to the clinical field.
The purposes of this review were (1) to compile, analyze, and summarize the data available in the literature regarding experimental studies in animals that used biologic methods against a control group that resulted in a decreased rate of OTM or its inhibition; (2) to compare the different methods and their outcomes; and (3) to give the clinician a clear overview of the scientific evidence available in the literature with a quality analysis of the methodologies used in the articles reviewed, thus facilitating research for professionals with an interest in this area. The main specific questions asked in this review were the following: Which experimental biologic methods have a decreasing or inhibitory effect on OTM? How efficient are these methods?
Material and methods
Protocol
The structure of the review protocol was developed before the start of the study, and the reporting of findings followed the PRISMA guidelines ( www.prisma-statement.org ). Because the experimental studies on which this systematic review was based were on animals, our protocol could not be registered in the PROSPERO database.
Information resources
A search was made of the MedLine (Entrez PubMed, www.ncbi.nim.nih.gov ), SCOPUS ( www.scopus.com ), and Web of Science ( www.isiknowledge.com ) databases to find possible studies matching our established selection criteria, including all articles published up to January 21, 2016. We searched for gray literature by exploring the OpenGrey database, European Association for Grey Literature Exploitation, also up to January 21, 2016, without applying language restrictions.
Search strategy
Our search strategy used the medical subject heading term “tooth movement” crossed with “inhibition,” “inhibit,” or “decrease” and excluded the terms “relapse” or “increase” or “enhance” or “promotion.” Supplementary Table I summarizes the full search strategy, including animal search filters, in all databases used. Some main orthodontic journals not indexed in the Journal Citation Report index were also hand searched to identify potential studies not found in the electronic search ( Supplementary Table I ).
Eligibility
Articles selected for this study fulfilled the following criteria for inclusion, according to the PICOS format.
- 1.
Population: animals; any experimental study or clinical investigation that included at least 1 experimental group with a minimum of 5 animals or samples per group.
- 2.
Intervention: biologic methods of decreasing or inhibiting tooth movement using orthodontic or orthopedic devices to apply forces.
- 3.
Comparison: control group without a biologic method.
- 4.
Outcome: rate of OTM deceleration or inhibition.
- 5.
Study design: experimental controlled trials.
Excluded from the selection were case reports, case series, descriptive studies, review articles, opinion articles, letters, and articles that did not correspond to the objectives of this review or did not have an adequate description of the technique or the administration dose.
Study selection
Eligibility was assessed by 2 observers (M.C-P. and R.M.Y-V.) acting independently. Articles were initially selected on the basis of the title and abstract, with the complete article reviewed whenever there was doubt about whether it should be included. Disagreements were resolved by consensus or by a third experienced reviewer who was requested to arbitrate (A.I-L.). After the 2 reviewers had separately applied the inclusion and exclusion criteria to each article, concordance between them was measured using the kappa index.
Data collection and analysis
Data were extracted by 1 observer (M.C-P.). A data extraction sheet was developed and piloted. Conflicts during data collection were resolved by discussion with a second (R.M.Y-V.) or a third experienced observer (A.I-L.). Data were extracted for the following items: author and year, study design, sample (size, species, age, and sex), a brief description of the methods, applied force, total treatment or experimentation time, decrease in the rate of OTM, and clinical applicability.
Methodologic quality and risk of bias of individual studies
The methodologic quality of the selected articles was assessed using the Methodological Index for Non-Randomized Studies (MINORS). The 12 variables analyzed were clearly stated: aim, inclusion of consecutive patients, prospective collection of data, end points appropriate to the aim of the study, unbiased assessment of the study end point, follow-up period appropriate to the aim of the study, loss to follow-up less than 5%, prospective calculation of the study size, adequate control group, contemporary groups, baseline equivalence of the groups, and adequate statistical analysis. After this analysis, every item scored 0 when not reported, 1 when it was reported but inadequate, and 2 when it was reported and adequate. Articles obtaining between 0 and 7 points were assessed as low quality and therefore had a high risk of bias; studies with 8 to 15 points were considered as medium quality and with a medium risk of bias; and articles obtaining 16 to 24 points were classed as high quality and with a low risk of bias.
Results
Study selection
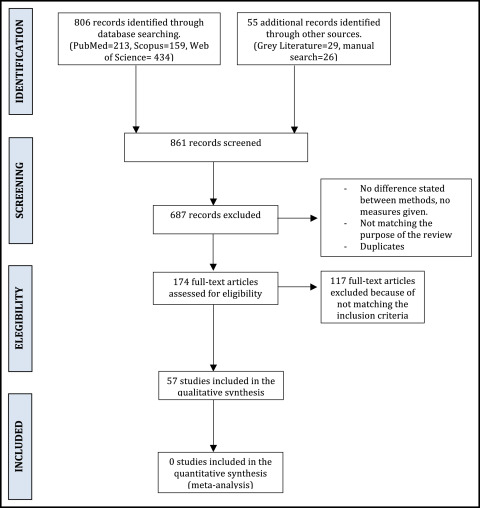
Two independent observers selected the studies, and good concordance was shown (kappa index, 0.8). A complete flow diagram of the search is given in the Figure .
Study characteristics and quality assessment
Rats were the most frequently used models in the sample (38 of 57 articles: 25 Wistar, 13 Sprague Dawley), followed by mice (9 of 57) and rabbits (4 of 57); guinea pigs and dogs were used in 2 articles each, and cats and monkeys in 1 each. The sample sizes seldom exceeded 25 subjects per group (6 of 57 articles). Randomization of the samples was mentioned in 24 articles, and blinding measures were found in only 14 studies ( Table I ).
| Authors, year | Clearly stated aim | Inclusion of consecutive sample | Prospective collection of data | End points appropriate to aim of study | Unbiased assessment of study end point | Follow-up period appropriate to aim of study | Loss to follow-up less than 5% | Prospective calculation of study size | Adequate control group | Contemporary groups | Baseline equivalence | Adequate statistical analysis | Total | Q | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical methods | |||||||||||||||
| Olteanu et al, 2015 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 14 | M | M |
| Kanzaki et al, 2015 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 13 | M | M |
| Hakami et al, 2015 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 14 | M | M |
| Fernandez-González et al, 2015 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 20 | H | L |
| Venkataramana et al, 2014b | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 13 | M | M |
| Venkataramana et al, 2014a | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 13 | M | M |
| Oliveira et al, 2014 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Nagaie et al, 2014 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 1 | 1 | 13 | M | M |
| Toro et al, 2013 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | M | M |
| Kaipatur et al, 2013 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 18 | H | L |
| Yabumoto et al, 2013 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | M | M |
| Olyaee et al, 2013 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Sodagar et al, 2013 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Kondo et al, 2013 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 1 | 14 | M | M |
| Esfahani et al, 2013 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | M | M |
| Yoshimatsu et al, 2012 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Kohara et al, 2012 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 1 | 13 | M | M |
| Hammad et al, 2012 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 13 | M | M |
| Meh et al, 2011 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Hao and Hua, 2011 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Gonzales et al, 2011 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Shoji et al, 2010 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 13 | M | M |
| Santos et al, 2010 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Han et al, 2010 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Choi et al, 2010 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Baysal et al, 2010 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Akhoundi et al, 2010 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Karras et al, 2009 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Fujimura et al, 2009 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | M | M |
| Sprogar et al, 2008 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Kriznar et al, 2008 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Kitaura et al, 2008 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Hauber Gameiro et al, 2008 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Sprogar et al, 2007 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Keles et al, 2007 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 14 | M | M |
| Dunn et al, 2007 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| de Carlos et al, 2007 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 2 | 17 | H | L |
| Bildt et al, 2007 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| de Carlos et al, 2006 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Arias and Marquez-Orozco, 2006 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Jäger et al, 2005 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 17 | H | L |
| Liu et al, 2004 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Gurton et al, 2004 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 2 | 15 | M | M |
| Shirazi et al, 2002 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 16 | H | L |
| Zhou et al, 1997 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 15 | M | M |
| Karsten and Hellsing, 1997 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 0 | 2 | 18 | H | L |
| Kehoe et al, 1996 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 13 | M | M |
| Igarashi et al, 1994 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Wong et al, 1992 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 13 | M | M |
| Hellsing and Hammarstrom, 1991 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 0 | 2 | 18 | H | L |
| Mohammed et al, 1989 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Chumbley and Tuncay, 1986 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 13 | M | M |
| Sandy and Harris, 1984 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 2 | 0 | 2 | 14 | M | M |
| Low-level laser therapy | |||||||||||||||
| Kim et al, 2015 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
| Kim et al, 2009 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Seifi et al, 2007 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 15 | M | M |
| Gene therapy | |||||||||||||||
| Kanzaki et al, 2004 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 16 | H | L |
The most commonly used biologic method leading to reduced orthodontic tooth movement was pharmacologic administration, and a wide range of substances were used (53 of 57 articles). The main substances evaluated in the selected articles were nonsteroidal anti-inflammatory drugs (NSAIDS) in 14 articles, followed by cytokines and bisphosphonates in 10 articles each. Three articles were based on antihistamines, and 2 articles each on hormones, fluoride, and beta blockers. Finally, 10 articles concentrated on other substances, such as immunosuppressants, morphine, nitric oxide, phenytoin, nicotine, simvastatin, endothelin, and tetracycline, or Nrf2 activators. Twenty-five studies were categorized as high quality with a low risk of bias (over 16 points), although on the 24-point scale used, no article scored more than 20 points. The remaining articles were considered to be medium quality.
Outcomes
The methods included in this review fall into 3 major categories: chemical or pharmacologic methods, gene therapy, and low-level laser therapy ( Table II ). Fifty-three of the 57 studies were classed as chemical methods. These comprised a wide range of substances, ranging from NSAIDs to bisphosphonates and cytokines, and were mainly administered in 1 of 2 forms: local or systemic. NSAIDS and other selective COX-2 inhibitors were the most frequently described substances (14 of 53 studies). Diclofenac seems to have the strongest inhibitory effect on OTM; de Carlos et al, in a study rated as high quality, described complete inhibition when diclofenac was used on rats under 100 and 50 g of force ( P <0.01), and partial inhibition with rofecoxib under 50 g of force ( P <0.01), although some movement was found (a reduction of approximately 70%) with rofecoxib and 100 g of force, compared with the controls ( P <0.05). In another article by the same authors, no statistically significant reduction in OTM was found with parecoxib or celecoxib compared with the controls at 50 g of force. However, short-term use of celecoxib slowed down OTM by 30% and long-term use by 46% ( P <0.01), as determined by a study of high methodologic quality, whereas in another, assessed as medium quality, 50% less tooth movement was found with the same drug ( P <0.01). However, a higher decrease in OTM was found by Hammad et al with paracetamol and ketorolac than with celecoxib ( P <0.01). Indomethacin obtained different results; it was reported to reduce the rate of OTM by half compared with the controls ( P <0.01), as described in a study rated as high quality, and to reduce OTM by 40% ( P <0.05) and 25% ( P <0.01) in 2 studies of medium methodologic quality. In the latter study, the prostaglandin (PG) analogs, prostacyclin (PGI2) and thromboxane A2 (TxA2), were compared with 2 PG inhibitors, indomethacin (a PGI2 inhibitor) and imidazole (a TxA2 inhibitor). The PG analogs—PGI2 much more than TxA2—significantly increased the rate of OTM in rats, whereas indomethacin and imidazole reduced OTM, with no significant differences in their inhibitory effects. Leukotriene inhibitors—and not only PG—were also reported to decrease OTM (application of AA861 resulted in 29.8% less OTM and 36% less tooth movement combined with indomethacin) ( P <0.05), as 1 high-quality study demonstrated ( Table II ).
| Authors, year | Study design | Sample (n) | Description of groups | Species | Age, sex | Force (g) |
Time (d) | Decrease in rate of OTM | Human clinical applicability tested |
|---|---|---|---|---|---|---|---|---|---|
| Chemical methods | |||||||||
| Olteanu et al, 2015 | CS | 24 | G1) CG: OTM only; G2) 1.5 mL aspirin by gastric gavage + OTM; G3) 1.2 mL algocalmin by gastric gavage + OT | WR | NM, 24 M | 25 | 28 | Decrease of OTM with aspirin and algocalmin ( P = 0.0001) | Yes |
| Kanzaki et al, 2015 | CS | 20 | G1) CG: no OTM; G2) OTM + right hemi-maxilla: 2 μL intragingival SFN (Nrf2 activator) in DMF, left hemi-maxilla DMF only; G3) OTM + right hemi-maxilla: 2 μL intragingival EGCG (Nrf2 activator) in DMF, left hemi-maxilla DMF only | Mi | 6 wk, 20 M | 10 | 21 | 60% less OTM compared with controls ( P <0.05) | No |
| Hakami et al, 2015 | CS | 32 | G1) CG: no OTM; G2) 1.5 μg/d intragingival IL-4 + OTM; G3) intragingival PBS + OTM; G4) 0.015 μg/d intragingival IL-4 + OTM; G5) 0.15 μg/d intragingival IL-4 + OTM | Mi | 10-12 wk, 32 M | 10 | 12 | Less OTM with 1.5 μg/d IL-4 but not with the rest of the doses ( P <0.01) | No |
| Fernandez-González et al, 2015 | CS | 42 | G1) CG: OTM only; G2: OTM + twice/w subgingival injections of 5 mg/kg OPG-Fc mesial and distal to maxillary first molar | SDR | 6 mo, 42 M | 50 | 21 | 52%, 31%, and 22% less OTM at days 7, 14 ( P <0.01), and 21 ( P <0.05), respectively | Yes |
| Venkataramana et al, 2014b | CS | 20 | G1) CG: 1 mL IP saline solution + OTM; G2) 1.5 mg/kg IP pamidronate + OTM | R | 16 wk, 20 M | 100 | 21 | Less OTM with pamidronate ( P <0.05) | Yes |
| Venkataramana et al, 2014a | CS | 20 | G1) CG: 1 mL IP saline solution + OTM; G2) 0.3 mg/kg intragingival ibandronate + OTM | R | 16 wk, NM | 100 | 21 | Less OTM with ibandronate ( P <0.05) | Yes |
| Oliveira et al, 2014 | CS | 20 | G1) vehicle only; G2) OTM + vehicle; G3) OTM + 0.1 mg/kg/d oral propranolol; G4) OTM + 20 mg/kg/d oral propranolol | WR | 3 mo, 20 M | 50 | 10 | Low dose propranolol (0.1 mg/kg) reduced OTM by 41%; a higher dose did not ( P <0.05) | No |
| Nagaie et al, 2014 | CS | 20 | G1) CG, OTM + oral administration of basal pure water; G2) OTM + 13 μg/mL TSC water containing 1 μg/mL nicotine | WR | 13 wk, 20 M | NM | 10 | 28% less OTM with nicotine ( P <0.01) | No |
| Toro et al, 2013 | CS | 30 | G1) 2-wk SC injection vehicle then OTM; G2) 2-wk SC injections 25 mg/kg BE, then OTM; G3) 2-wk SC injections 1 mg/kg alendronate, then OTM | SDR | NM, 30 M | 13 | 28 | BE and alendronate reduced OTM by 64% and 84%, respectively ( P <0.05) | No |
| Kaipatur et al, 2013 | CS | 20 | G1) OTM + SC injection 0.015 mg/kg alendronate; G2) OTM + vehicle; G3) 3-mo alendronate, then OTM; G4) 3-mo vehicle, then OTM | SDR | 12 wk, 20 F | 50 | 56 | 65% less OTM on G1/G2 ( P = 0.05), 86% less OTM on G3/G4 (prior intake bisphosphonates) ( P <0.01) | No |
| Yabumoto et al, 2013 | CS | 80 | G1) wild-type mice, OTM + saline solution; G2) wild-type mice, OTM + injection of 15 mg/kg IP reveromycin A; G3) OPG deficient KO mice, OTM + saline solution; G4) OPG deficient KO mice, OTM + 15 mg/kg IP reveromycin A | Mi | 8 wk, 80 M | NM | 3 | NSRD after 3 days between groups | No |
| Olyaee et al, 2013 | CS | 48 | G1) OTM + 100 mg/kg/d ethinyl estradiol by gavage + 1 mg/kg/d norgestrel by gavage; G2) CG OTM + saline solution | WR | 12 wk, 48 F | 30 | 14 | 39% less OTM with estradiol and norgestrel ( P <0.05) | No |
| Sodagar et al, 2013 | CS | 28 | G1) OTM only; G2) OTM + injection of 0.3 mg celecoxib | SDR | 5 wk, 28 M | 60 | 18 | 50% less OTM ( P <0.01) | No |
| Kondo et al, 2013 | CS | NM | G1) 7-d IP injections 20 μg/g propranolol (β-antagonist) then OTM; G2) 7-d IP injections 5 μg/g isoproterenol (β-agonist) then OTM; G3) 7-d IP injections 0.9% saline solution, then OTM | Mi | 8 wk, M | NM | 5 | β-antagonist decreased OTM by 35.7%; β-agonist increased it by 14.3% ( P <0.05) | No |
| Esfahani et al, 2013 | CS | 32 | G1) CG: OTM + saline solution; G2) OTM + 2.5 mg/kg IP simvastatin | WR | 8-10 wk, 32 M | 60 | 17 | Significantly less OTM ( P <0.024) | No |
| Yoshimatsu et al, 2012 | CS | 32 | G1) no OTM or injection (CG); G2) OTM + 0.015 mg/d IL-12; G3) OTM + 0.15 mg/d IL-12; G4) OTM + 1.5 mg/d IL-12 G5) OTM + PBS | Mi | 8 wk, 32 M | 10 | 12 | 1.5 mg/d IL-12 significantly decreases OTM ( P <0.001); with lower doses, NSRD were found | No |
| Kohara et al, 2012 | CS | NM | G1) OTM + injections IFN-g (0.015-1.5 μg per 20 mL); G2) (CG) OTM + PBS | Mi | 8 wk, NM | 10 | 12 | 61.4% less OTM in the group treated with interferon ( P <0.05) | No |
| Hammad et al, 2012 | CS | 40 | G1) CG: reverse osmosis water + OTM; G2) 10 mg/kg celecoxib + OTM; G3) 3 mg/kg ketorolac + OTM; G4) 150 mg/kg paracetamol + OTM. | WR | 12 wk, 40 M | 50 | 60 | celecoxib did not reduce OTM compared with ketorolac and paracetamol ( P <0.01) | Yes |
| Meh et al, 2011 | CS | 48 | G1) oral saline solution; G2) OTM + oral saline solution; G3) 3 mg/kg/d oral cetirizine + OTM | SDR | 13 wk, 48 M | 25 | 42 | cetirizine reduced OTM by 26.1% ( P <0.01) | No |
| Hao and Hua, 2011 | CS | 72 | G1) OTM + local injections of PBS (CG); G2, G3, G4) OTM and systemic injections of 0.16, 0.12, 0.08, and 0.04 mg/mL rhsTNF-RI. | SDR | 6 wk, 72 M | 50 | 14 | NSRD in the 0.04 mg/mL group, but 40% decrease in OTM in the rest of the groups ( P <0.01) | No |
| Gonzales et al, 2011 | CS | 50 | G1) no OTM, no NaF; G2) OTM, no NaF; G3) OTM + 2-wk 45 ppm oral NaF; G4) OTM + 4-wk 45 ppm oral NaF; G5) OTM + 12-wk 45 ppm oral NaF | WR | NM, 50 M | 50 | 84 | 74% decrease in OTM ( P <0.01) | Yes |
| Shoji et al, 2010 | CS | 48 | in OPG -/- mice (a model for juvenile Paget disease): G1) CG: IP saline solution + OTM; G2) 1.25 mg/kg/d IP alendronate + OTM in wild-type mice; G3) CG: IP saline solution + OTM; G4) 1.25 mg/kg/d IP alendronate + OTM | Mi | 8 wk, 48 M | NM | 3 | administration of alendronate to OPG -/- mice decreased OTM ( P <0.01) | No |
| Santos et al, 2010 | CS | 120 | G1) OTM + saline solution; G2) OTM + FK506; G3) FK506 only; G4) saline solution only | WR | 9 wk, 120 M | 35 | 14 | 19% less OTM with tacrolimus immunosuppressant (FK506) treatment ( P <0.05) | No |
| Han et al, 2010 | CS | 32 | G1) 2.5 mg simvastatin per kg/d; G2) CG | WR | 7-8 wk, 32 M | 50 | 49 | 45% less OTM ( P <0.001) | Yes |
| Choi et al, 2010 | CS | 54 | G1) 2.5 mM/L clodronate; G2) 10 mM/L clodronate; G3) CG | WR | 8 wk, 27 M/27 F | 60 | 17 | OTM reduced by 32% and 36.3%, respectively ( P <0.05) | Yes |
| Baysal et al, 2010 | CS | 28 | G1) no OTM; G2) OTM + thyroxine; G3) OTM + doxycycline; G4) OTM only | WR | 7-8 wk, 28 M | 50 | 14 | NSRD | Yes |
| Akhoundi et al, 2010 | CS | 40 | G1) OTM; G2) OTM + injections 5 mg/d morphine; G3) OTM + 5 mg/d morphine + 20 mg/d naltrexone; G4) OTM + 20 mg/d naltrexone/normal saline solution | WR | NM, 40 M | 60 | 14 | morphine reduced OTM by 52% ( P <0.05) | No |
| Karras et al, 2009 | CS | 50 | G1) CG, OTM only; G2) OTM + injections of alendronate sodium of 7 mg/kg body weight per week | SDR | NM, NM | 50 | 35 | 42% less OTM in the alendronate group after 4 weeks ( P <0.001) | No |
| Fujimur et al, 2009 | CS | NM | G1) OTM + local bisphosphonate; G2) OTM + PBS | Mi | 8 wk, M | NM | 12 | 50% less OTM ( P <0.05) | No |
| Sprogar et al, 2008 | CS | 34 | G1) no OTM + saline solution; G2) OTM + saline solution; G3) OTM + 10 mg/kg IP famotidine | WR | NM, 34 M | 25 | 42 | significantly less OTM ( P <0.001) | No |
| Kriznar et al, 2008 | CS | 34 | G1) no OTM + saline solution; G2) OTM + saline solution; G3) OTM + 10 mg/kg IP cetirizine | WR | NM, 34 M | 25 | 42 | significantly less OTM ( P <0.05) | No |
| Kitaura et al, 2008 | CS | NM | G1) OTM + daily injection 10 μg anti-CFms antibody; G2) OTM + PBS | Mi | 8 wk, NM | 10 | 12 | anti-CFms antibody reduces OTM by in 36.7% ( P <0.05) | No |
| Hauber Gameiro et al, 2008 | CS | 32 | G1) OTM + IP injections celecoxib 3 d; G2) OTM + IP injections saline 3 d; G3) OTM + IP injections celecoxib 14 d; G4) OTM + IP injections control 14 d | WR | NM, 32 M | 50 | 14 | celecoxib decreases OTM by 30% with short-term dosage, and 46% with long-term dosage ( P <0.05) | No |
| Sprogar et al, 2007 | CS | 30 | G1) OTM + TBC3214; G2) OTM + placebo; G3) placebo | WR | 11-12 wk, 30 M | 25 | 40 | daily TBC3214, treatment reduces OTM by 33.3% ( P <0.001) | No |
| Keles et al, 2007 | CS | 51 | G1) OTM; G2) Pamidronate + OTM; G3) OPG + OTM | Mo | 8 wk, 51 M | 22,4 | 12 | pamidronate inhibited OTM by 34%, OPG by 77% ( P <0.01) | Yes |
| Dunn et al, 2007 | CS | 30 | G1) OTM + injection PBS; G2) OTM + 0.5 mg/kg local injections recombinant OPG; G3) OTM + 50 mg/kg local injections recombinant OPG | WR | NM, 30 M | 54 | 21 | OTM was inhibited by 70.6% after 14 days in higher-dose group ( P <0.001) and by 31.8% in lower-dose group ( P <0.05) | No |
| de Carlos et al, 2007 | CS | 28 | G1) OTM + rofecoxib; G2) OTM + celecoxib; G3) OTM + Ppecoxib; G4) control | WR | 12 wk, 28 M | 50 | 5 | rofecoxib inhibits OTM ( P <0.05); NSRD between parecoxib and celecoxib and controls | Yes |
| Bildt et al, 2007 | CS | 18 | G1) OTM + PBS injection; G2) OTM + injection 6 mg CMT-3/kg body weight; G3) OTM + injection 30 mg CMT-3/kg body weight | WR | NM, 18 M | 10 | 14 | CMT reduced OTM by 15.7% in 6-mg group and 34.3% in 30-mg group, respectively ( P <0.05) | No |
| de Carlos et al, 2006 | CS | 42 | G1) OTM (50 g) + rofecoxib; G2) OTM (50 g) + diclofenac; G3) control G4) G5) G6) OTM (100 g) + same pharmacologic treatment as 1, 2, and 3 |
WR | NM, 42 M | 50-100 | 10 | Diclofenac inhibits OTM under 50 and 100 g forces ( P <0.01); rofecoxib inhibits OTM under 50 g force ( P <0.01) and reduces it by 73.6% under 100 g force ( P <0.05) | Yes |
| Arias and Marquez-Orozco, 2006 | CS | 36 | G1) OTM + 100 mg/kg/d ASA; G2) OTM + 30 mg/kg/d ibuprofen; G3) OTM + 200 mg/kg/d acetaminophen; G4) CG: OTM + vehicle | WR | NM, 36 M | 30 | 10 | aspirin reduced OTM by 38.75% ( P <0.05), ibuprofen by 41.52% ( P <0.01); acetaminophen did not affect OTM | Yes |
| Jäger et al, 2005 | CS | 80 | G1) OTM + PBS; G2) OTM + sIL-1-R; G3) OTM + sTNF-a-RI; G4) OTM + both | WR | 12 wk, 80 M | 50 | 12 | 60% less OTM ( P <0.05) | No |
| Liu et al, 2004 | CS | 26 | G1) OTM; G2) OTM + 2.5 mM clodronate; G3) OTM + 10 mM clodronate; G4) OTM + 40 mM clodronate | WR | 7 wk, 26 M | 12 | 21 | 56%, 65%, and 81% less OTM, respectively ( P <0.001) | No |
| Gurton et al, 2004 | CS | 150 | G1) OTM + iloprost; G2) OTM + indomethacin; G3) OTM + U 46619; G4) OTM + imidazole; G5) OTM + 0.9% NaCl; G6) no OTM + NaCl; G7) no OTM or solution | SDR | NM 150 M | 20 | 5 | Pg analogs increase OTM by 31.28% Pg antagonists reduce OTM by 20.26% ( P <0.01) |
Yes |
| Shirazi et al, 2002 | CS | 48 | G1) CG, no injections; G2) saline solution group; G3) 200 mg/kg injections of L-arg; G4) 10 mg/kg L-NAME group | SDR | NM, M | 60 | 13 | 50% less OTM with the L-NAME group (reduced nitric oxide production led to a decrease in OTM) ( P <0.001) | No |
| Zhou et al, 1997 | CS | 96 | G1) OTM + subcutaneous indomethacin; G2) OTM + saline solution | SDR | 5-6 wk, 96 M | 40 | 10 | 40% less OTM with indomethacin ( P <0.05) | No |
| Karsten and Hellsing, 1997 | CS | 20 | G1) OTM + phenytoin; G2) control | SDR | 3-5 mo, 20 F | 15 | 42 | not conclusive. | No |
| Kehoe et al, 1996 | CS | 40 | G1) CG: OTM + placebo; G2) 100 μg/kg/12-h misoprostol + OTM; G3) 200 mg/kg/12-h acetaminophen + OTM; G4) 30 mg/kg/12-h ibuprofen + OTM | GP | 6-8 wk, 40 M | 25 | 11 | acetaminophen showed NSRD with controls; ibuprofen reduced OTM by approximately 22% ( P <0.001) | No |
| Igarashi et al, 1994 | CS | 77 | G1) systemic AHBuBP (bisphosphonate) every 24 h + OTM; G2) OTM only; G3) topical AHBuBP + OTM | WR | 9-10 wk, 77 M | 16.8 | 21 | 40% less OTM with systemic application, 70% less OTM with topical administration ( P <0.001) | No |
| Wong et al, 1992 | CS | 11 | G1) CG: OTM + sodium bicarbonate; G2) OTM + oral administration of 65 mg/kg/d ASA | GP | NM, NM | 8 | 28 | NSRD | No |
| Hellsing and Hammarstrom, 1991 | CS | 16 | G1) OTM in pregnant rats; G2) OTM in nonpregnant rats; G3) nonpregnant rats + NaF | SDR | 3-5 mo, 16 F | 15 | 21 | 52% less OTM with NaF; 39% more OTM in pregnant rats ( P <0.01) | No |
| Mohammed et al, 1989 | CS | 132 | G1) OTM + leukotriene synthesis inhibitor; G2) OTM + indomethacin; G3) OTM; G4) OTM + both groups | SDR | NM, NM | 60 | 14 | 29.8% less OTM with AA861 combined with indomethacin; 36.2% less OTM with indomethacin only ( P <0.05) | No |
| Chumbley and Tuncay, 1986 | CS | 12 | G1) OTM (CG); G2) OTM + oral administration of 5 mg/kg/d indomethacin | C | 12-18 mo, NM | 250 | 21 | indomethacin group achieved approximately 50% less than the CG ( P <0.01) | No |
| Sandy and Harris, 1984 | CS | 14 | G1) CG (OTM + vehicle of MC; G2) OTM + flurbiprofen | R | NM 7 F/7 M | 100 | 14 | NSRD | No |
| Gene therapy | |||||||||
| Kanzaki et al, 2004 | CS | 20 | G1) CG, no OTM CG; G2) OTM + PBS; G3) OTM + injections of OPG gene transfer | WR | 6 wk, 20 M | 17 | 20 | 92.8% less OTM ( P <0.001) | Yes |
| Low-level laser therapy | |||||||||
| Kim et al, 2015 | CS | 10 | G1) CG: OTM alone; G2) OTM into the grafted defects; G3) OTM into the grafted defects + LLLT. | D | 18-24 mo, 10 M | 100 | 42 | LLLT decreased OTM into the bone-grafted surgical defects ( P <0.01) | Yes |
| Kim et al, 2009 | CS | 12 | G1) CG OTM only; G2) OTM + CO; G3) OTM + LLLT; G4) OTM + CO + LLLT | D | 84 wk, 12 M | 150 | 56 | LLLT after CO decreases OTM 48.4% ( P <0.001) | No |
| Seifi et al, 2007 | CS | 18 | G1) CG; G2) LLLT (850 nm); G3) LLLT (630 nm) | R | 16 wk, 18 M | 10-120 | 16 | Pulsed and continuous LLLT reduced OTM by 40.6% and 50.6% ( P <0.001) | No |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


