Introduction
Antibacterial adhesives were developed to reduce the incidence of white spot lesions in orthodontic patients. Compounds that contain triazine are known as effective antibacterial agents. The aims of this study were to develop an experimental orthodontic adhesive containing 1,3,5-triacryloylhexahydro-1,3,5-triazine (TAT) and to characterize it.
Methods
TAT was added in 3 concentrations (10%, 15%, and 20%) to the experimental orthodontic adhesive. Antibacterial activity was assayed by brain-heart infusion broth dilution against Streptococcus mutans . The degree of conversion was measured using Fourier transform infrared spectroscopy, and solvent degradation was evaluated by Knoop microhardness before and after immersion in ethanol for 2 hours. The shear bond strength of metal brackets bonded to bovine enamel surface was assessed.
Results
All experimental adhesives reduced bacterial growth. The addition of 15% and 20% TAT increased the degree of conversion compared with the control group (0%) and the 10% group. All groups showed a decrease in hardness after ethanol immersion, and there was also a decrease in the percentage of variation of Knoop hardness in the experimental adhesives containing TAT, whereas the shear bond strength increased.
Conclusions
Orthodontic adhesives containing TAT are promising antibacterial materials, especially those with 15% and 20% TAT.
Highlights
- •
Orthodontic adhesive system with a triazine-based monomer had antibacterial activity.
- •
Adding this to the adhesive increased the degree of conversion and shear bond strength.
Orthodontic patients experience a higher cariogenic challenge because of bacterial biofilm accumulation around their brackets. An increase in oral colonization by Streptococcus mutans lowers the pH, and the demineralization process that takes place leads to the development of white spot lesions. Proper oral hygiene is the foremost mode to prevent lesions, but many patients are inefficient at this. Thus, the development of antibacterial adhesives could help to overcome this problem. However, the incorporation of antibacterial compounds should not decrease material properties.
Seeking the improvement of orthodontic adhesives, some authors have performed studies using different antibacterial agents such as MDPB, chlorhexidine, nanoparticle silver, triclosan, zinc oxide, titanium dioxide, and others. A reduction in bacterial growth is observed with a decrease in the adhesive’s properties, such as lower bond strength. Addition of MDPB monomer causes reliable antibacterial growth without significantly compromising the bond strength because of the copolymerization of the methacrylate radical with other monomers of the adhesive. Studies have shown that other methacrylated-based monomers have antibacterial properties. Triazine compounds have been synthesized and evaluated in the medical field as antibacterial, antiviral, antimalarial, antiprotozoal, and anticancer agents. They are also used in the treatment of depression. 1,3,5-triazine can decrease bacterial growth because it is a small compound that mimics the hydrophobic and charge patterns detected in the pharmacophore of short cationic antimicrobial peptides. It is more selective against gram-positive bacteria, by means of membrane integrity disruption, and shows low hemolytic activity.
To date, no study has been performed with triazine compounds as the antibacterial agent in orthodontic adhesive systems. The influence of triazine incorporation in orthodontic adhesive materials properties (eg, degree of conversion, antibacterial activity, and bond strength) should be investigated. The aims of this study were to develop an experimental orthodontic adhesive and to evaluate the influence of the addition of 1,3,5-triacryloylhexahydro-1,3,5-triazine (TAT) on the adhesive’s properties.
Material and methods
The experimental orthodontic adhesives were formulated: 75 wt% bisphenol A glycidyl methacrylate and 25 wt% of triethylene glycol dimethacrylate, 1% mol of camphorquinone, ethyl-4-dimethylamino benzoate, and diphenyliodonium hexafluorophosphate as the photoinitiator system, and 0.1 wt% of butylated hydroxytoluene, all from Sigma-Aldrich (St Louis, Mo). Also, 5% of colloidal silica (AEROSIL 200; Evonik, Piscataway, NJ) was added. TAT (Sigma-Aldrich) was added in 3 concentrations: 10%, 15%, and 20%; for the control, a group with no TAT was used. The light source device used for photoactivation was Radii cal (1200 mW/cm 2 ; SDI, Bayswater, Victoria, Australia).
The specimens of each group were prepared (2 × 2 × 3 mm), photopolymerized for 40 seconds, and submitted to ethylene oxide sterilization. Each specimen was placed in a well of a sterile 96-well plate. Each well contained 300 μL of brain-heart infusion broth (Sigma-Aldrich) with 1% sucrose and 20 μL of a suspension of an overnight broth culture of S mutans UA 159 adjusted to optical density of 0.67 (550 nm). The 96-well plate was incubated at 37°C for 24 hours, and 100 μL of each well was diluted in 900 μL of saline solution until the 10 −6 dilution. Two 25-μL drops of each dilution were platted in brain-heart infusion agar Petri dishes and incubated for 48 hours at 37°C. The number of colony forming units (CFUs) was visually counted by optical microscopy and transformed to log CFU per milliliter.
The degree of conversion was evaluated through Fourier transform infrared spectroscopy (Vertex 70; Bruker Optics, Ettlingen, Germany). The spectrometer was equipped with an attenuated total reflectance device (Platinum ATR-QL; Bruker Optics). A support device was used to hold the light-curing unit at a standard distance of 5 mm from the sample for photopolymerization of 40 seconds. The absorbance spectra were obtained before and immediately after polymerization using software (Opus 6.5; Bruker Optics), transferred to ImageJ software (Image J 1.47; National Institutes of Health, Bethesda, Md), and the degree of conversion was calculated.
For the solvent degradation, the specimens from each group were prepared (6 mm in diameter × 1 mm in thickness) and embedded in acrylic resin (VIPIFlash; Vipi Industry, Pirassununga, São Paulo, Brazil). The samples were polished with silicon carbide sandpapers (600, 1200, and 2000 granulation) and felt disks saturated with alumina suspension (alumina, 6 μm; Arotec, Cotia, São Paulo, Brazil). Three indentations were made (at 10 g for 5 seconds) in each specimen with a microhardness tester (HMV 2; Shimadzu, Tokyo, Japan).
Knoop hardness values before and after the immersion in ethanol (Labsynth, Diadema, São Paulo, Brazil) for 2 hours were recorded. The percentage of variation of Knoop hardness was calculated for each specimen.
For the shear bond strength test, 48 crowns of extracted bovine incisors without fractures or cracks stored in distilled water at 4°C for less than 3 months were used in this study. The teeth were embedded in acrylic resin, and the facial surfaces were cleaned with fluoride pumice for 10 seconds and etched with 37% phosphoric acid gel (Atacktec; CaiTECH Indústria, Rio do Sul, Santa Catarina, Brazil) for 30 seconds, rinsed with water for 30 seconds, and dried with oil-free compressed air. Transbond XT Primer (3M Unitek, Monrovia, Calif) was applied to the bonding surface and photoactivated for 20 seconds. Maxillary central incisor metal brackets (Roth Max; Morelli, Sorocaba, São Paulo, Brazil) with a base area of 11.65 mm 2 were bonded to the teeth with each experimental orthodontic adhesive.
The experimental orthodontic adhesives were applied to the bracket base, and it was placed on the facial tooth surface. To standardize the thickness of the adhesive, brackets were placed under a load of 300 g, and the excess adhesive was removed. The adhesives were light-cured for 40 seconds (10 seconds for each side of the bracket). After 24 hours of storage in distilled water at 37°C, the specimens were submitted to a shear bond strength test in a universal testing machine (EMIC DL200; Equipamentos de Ensaio, São José dos Pinhais, Paraná, Brazil) using a knife-edged chisel with a crosshead speed of 1 mm per minute, and the results were recorded in megapascals.
The analysis of the residual adhesive on the tooth surface with the adhesive remnant index was realized with a stereoscopic microscope (10-times magnification), and the scores were recorded.
Statistical analysis
Statistical analysis was done with Sigma Plot for Windows (version 12.0; Systat Software, San Jose, Calif). The sample size calculation for each assay was based on previous studies.
Comparisons of antibacterial activity, degree of conversion, percentage of variation of Knoop hardness, and shear bond strength data were performed with 1-way analysis of variance and Tukey tests. Comparisons of initial and final Knoop hardness values were performed with paired t tests for each group.
Results
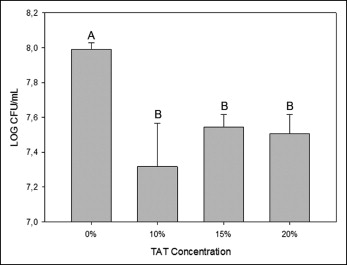
The addition of TAT to the experimental orthodontic adhesives showed a significant decrease in bacterial growth in comparison with the control group (7.99 ± 0.04 log CFU/mL) ( P <0.05). There was no statistical difference in bacterial growth as the concentration of TAT increased (7.32 ± 0.25, 7.54 ± 0.07, 7.51 ± 0.11 log CFU/mL, respectively, for 10%, 15%, and 20%) ( Fig 1 ).
The degrees of conversion of the groups are shown in Table I . There were significant increases in the degree of conversion of the experimental adhesives containing 15% (62.06% ± 1.26%) and 20% (62.70% ± 2.52%) TAT when compared with the control (57.08% ± 0.97%) ( P <0.05).
| Concentration | Degree of conversion (%), mean ± SD | Shear bond strength (MPa), mean ± SD |
|---|---|---|
| 0% (Control) | 57.08 ± 0.97 B | 11.15 ± 2.58 B |
| 10% | 57.59 ± 1.27 B | 15.00 ± 2.91 A |
| 15% | 62.06 ± 1.26 A | 15.51 ± 3.36 A |
| 20% | 62.70 ± 2.52 A | 14.44 ± 2.72 A |
For the solvent degradation, all experimental adhesives had similar initial Knoop hardness values and significant decreases in the final Knoop hardness after 2 hours of immersion in ethanol. There was a significant decrease of 18% in the percentage of variation of Knoop hardness of all experimental adhesives containing TAT ( P <0.05) compared with the control experimental adhesive (67.99 ± 1.97), and no statistical difference was observed among them ( Table II ).
| Concentration | KHN1 | KHN2 | ΔKHN% |
|---|---|---|---|
| 0% (Control) | 23.35 ± 3.36 A,a | 7.46 ± 1.06 b | 67.99 ± 1.97 B |
| 10% | 24.76 ± 1.38 A,a | 11.04 ± 0.88 b | 55.11 ± 3.03 A |
| 15% | 20.94 ± 3.35 A,a | 9.35 ± 2.69 b | 55.72 ± 5.83 A |
| 20% | 20.97 ± 2.46 A,a | 9.11 ± 1.64 b | 56.97 ± 3.16 A |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


