Introduction
For orthodontic applications, equiatomic nickel-titanium (NiTi) wires are used to level and align the teeth under bending conditions in the oral environment for long periods. The aim of study was to investigate the influence of bending stress on the nickel release of commercial NiTi orthodontic wires in vitro, simulating the intraoral environment as realistically as possible.
Methods
Two types of as-received orthodontic NiTi wires, free of performed internal stress, were immersed in artificial saliva. Half of the NiTi wires were exposed to continuous bending stress throughout the 14-day experimental period.
Results
The stressed NiTi wires exhibited substantial increases in the nickel release compared with the unstressed specimens during all experimental periods. The highest dissolution rate during the 0 to 1 day incubation period was observed for all stressed specimens. However, a slight increase of nickel released as a function of time was observed in the 3 groups of stressed specimens after 3 days of immersion. For the stressed specimens, it was hypothesized that the bending stress would induce buckling or cracking of the protective oxide film of the NiTi wires. In this study, the mechanism of nickel release was the underlying metal surface reacting with the surrounding environment.
Conclusions
The results indicated that bending stress influences the nickel release of NiTi wires. The factor of loading condition with respect to corrosion behavior and passive film should be considered in view of the widespread use of NiTi wires for dental devices.
Equiatomic nickel-titanium (NiTi) shape-memory alloys have been widely used for medical applications, because of their superelastic and shape-memory properties. For clinical applications, the mechanical property of NiTi could provide a constant stress over a wide range of strains and shapes. The constant stress allows the design of orthodontic archwires that generate the continuous and biomechanical forces that are communicated through the brackets for the leveling and alignment stages of tooth movement. During orthodontic treatment, the oral tissues can be exposed to fixed metal appliances, such as brackets and archwires. Metal appliances are prone to corrosion or degradation in the oral environment, which contains both organic species and inorganic components. The biocompatibility of a metallic implant is related to its resistance to corrosion, and the degradation of metal has been demonstrated to adversely affect biocompatibility. There is not total agreement on the biocompatibility of NiTi. Al-Waheidi reported on a case of severe allergic reaction to NiTi orthodontic wire in a patient allergic to nickel. Shih et al reported that both the supernatant and precipitate of the corrosive products of Nitinol wire were toxic to the primary cultured rat aortic smooth muscle cells. In the general population, nickel elicits adverse responses in 10% to 20% of sensitive patients. Concerns about nickel allergy are constantly rising in biomedical applications with nickel or nickel compounds.
The corrosion behavior of NiTi has been attributed to the stable passivating oxide layer, mainly composed of titanium and oxygen. The properties of the protective oxide film play an important role in corrosion resistance against an aggressive environment. The surface properties of NiTi critically depend on the procedure used for surface preparation. The effect of surface preparation, such as aging in boiling water, mechanical polishing, oxidizing in hydrogen peroxide, electropolishing on the oxide film of NiTi, was mentioned by Shabalovskaya. That article indicates that parameters such as oxidation media, temperature, and electrical potentials could produce either a titanium-base surface with titanium dioxide as the only oxide or a nickel-base surface with less stable nickel oxides and hydroxides. Other studies also reported the effect of surface treatment on the corrosion properties of NiTi alloy. Cisse et al reported that surface treatments such as heat treatment, mechanical polishing, electropolishing, and electropolishing followed by chemical passivation would change the surface oxide film of NiTi alloy and further alter the corrosion potential, corrosion rate, nickel release, and wettability. Man et al used the Nd-YAG laser to melt the surface of NiTi alloy, and the corrosion resistance of laser-treated specimens has been improved significantly because of increased amounts of titanium dioxide and the titanium-nickel ratio on the surface. Huang used the potentoidynamic test to evaluate the corrosion resistance of as-received NiTi orthodontic wires in artificial saliva, and the corrosion resistance was related to the preexisting surface defect and passive film.
For orthodontic applications, NiTi wires are subject to mechanical stresses and deformation for tooth movement. The applied forces might induce damage of the oxide film on the wire surface, and the loss of protection allows active metal to react with the surrounding environment. Also, NiTi repassivates slower than a cobalt-base alloy and titanium. The limited repassivation capacity of NiTi might release nickel ions into the oral cavity. Unfortunately, few published results are associated with the effect of mechanical stress on corrosion behavior or nickel ion release of NiTi. The role of the oxide film regarding nickel release from NiTi wires subjected to mechanical stress is still uncertain. The purpose of this investigation was to measure the nickel release and the surface properties of commercial NiTi orthodontic wires in artificial saliva under 3-point bending, simulating the intraoral environment as realistically as possible. The specimens were immersed in modified Fusayama artificial saliva with different acidities and maintained at 37°C for periods up to 14 days. The solutions were analyzed by an inductively coupled plasma mass spectrometer. Scanning electron microscope (SEM), scanning probe microscope (SPM), and Auger electron spectroscopy (AES) were used to measure the changes in surface properties after soaking in artificial saliva.
Material and methods
Two kinds of commercial NiTi orthodontic archwires (diameter, 0.016 in) were used in this investigation: Nitinol Classic (3M Unitek, Monrovia, Calif) and Sentalloy (Tomy, Fukushima, Japan). The as-received NiTi wires, obtained from the 2 straight sides of an archwire, free of performed internal stress, were ultrasonically cleaned in 95% alcohol. The chemical compositions of the NiTi wires were examined at 20 kV in an SEM (JSMR 840; JEOL Ltd., Tokyo, Japan) equipped with an energy-dispersive spectrometer (link AN 1000/85S; Oxford Instruments, Abingdon, Oxfordshire, United Kingdom). The chemical compositions were 50.5% titanium, 49.0% nicklel, and 0.5% chromium for as-received Nitinol wires and 48.2% titanium and 51.8% nickel for Sentalloy wires. We used 5 wires of each alloy in the energy-dispersive spectrometer analysis. Mechanical loading was determined by placing a wire in a 3-point bending apparatus attached to a universal testing machine (AG-1; Shimadzu, Kyoto, Japan). The length of the support (outer) span was 13 mm, which is the distance between the distal aspect of the bracket of a canine and the mesial aspect of the bracket of a second premolar. A loading rate of 0.2 mm per minute was used for the 3-point bending test. The nominal displacement of 3 mm corresponds to forces of 2.646 N for Nitinol wire and 2.401 N for Sentalloy wire at 37°C. We used 5 wires of each alloy in the mechanical tests.
A total of 8 groups (2 materials, 2 pH values, and 2 loading conditions) were investigated, and 8 specimens were prepared for each immersion test condition. All NiTi wires were ultrasonically cleaned in 95% alcohol and then rinsed with double-distilled water. The artificial saliva contained potassium chloride (0.4 g/L), sodium chloride (0.4 g/L), calcium chloride dihydrate (CaC1 2 )·2H 2 O (0.906 g/L), sodium phosphate monobasic dihydrate (NaH 2 PO 4 )·2H 2 O (0.690 g/L), sodium sulfide nonahydrate (Na 2 S)·9H 2 O (0.005 g/L), and urea (1 g/L). Except for the pH 5.3 condition, the solution was adjusted to pH 2 with lactic acid. Half of the specimens were exposed to continuous mechanical stresses to simulate an orthodontic archwire ligated to teeth in a dental arch. A 3-point flexure fixture, made of an acrylic sheet, was used to apply a continuous bending force deflected a distance of 3.0 mm ( Fig 1 ). The wires were fixed in the solution with a free length of 13 mm. The unbent specimens were also placed in the same designed vessel, and both end sides were not exposed to the artificial saliva solution. Additional control groups consisted of vessels with an identified volume of test solution (no NiTi wire). The integral dissolution protocol was selected to determine the dissolution kinetics by sequential measurement of nickel release into a solution of accumulated dissolution products. The results showed the integral dissolution rate. The test wire with an exposure area of 0.16 cm 2 was soaked in artificial saliva with a volume of 1.6 mL. The specimens were placed individually in vessels with a surface area to 37°C solution volume ratio of 0.1 cm to 1. After 1, 3, 7, and 14 days of immersion, the medium was poured from each vessel into a clean and labeled polypropylene tube that was then sealed, and each retrieved sample was cleaned and stored for surface analysis. The collected solutions were individually analyzed for nickel by using the inductively coupled plasma-mass spectrometer (model 4500 series; Hewlett Packard, Palo Alto, Calif). The detection limit of inductively coupled plasma-mass used in this study was 5 ppb for nickel ions. The statistical significances of the influence of the experimental factors were tested and verified by using 1-way analysis of variance and the post-hoc Turkey test at a level of confidence of 95% (version 15.0; SPSS, Chicago, Ill). Differences were considered significant at P <0.01.
For the surface analysis at the end of the immersion period, the specimens were removed, sonicated 3 times in double distilled water and once in absolute alcohol, dried with an argon gas stream, and immediately placed in a vacuum desiccator before surface examination by SEM, AES, and SPM. The composition of the reacted surface film was examined by AES (310 D; VG Microlab, East Sussex, United Kingdom). The vacuum pressure of AES was 2.9 × 10 −9 mm of mercury, and a monoenergetic electron beam with energy of 10 KV and current of 100 nA was used. Selected specimens for depth-profile examination were milled by argon ion bombardment at 3 KV and 9 mA. The surface topography of the NiTi wires was observed by using SPM (IIIa Dimension 3000; Digital Instruments, Tonawanda, NY).
Results
After soaking in artificial saliva at both pH 2 and pH 5.3, the mean values and standard deviations of the amounts of nickel ions released from all experimental groups are shown in Table I . The overall nickel amounts released from unstressed Nitinol and Sentalloy wires were extremely low and could not be detected during all immersion periods. In comparison with the unstressed wires, the stressed specimens had higher amounts of nickel ion release throughout the 14-day experimental period, and the results indicated that the bending stress induced higher nickel ion releases of NiTi wires compared with unstressed specimens in artificial saliva at both pH 2 and 5.3. In this study, pH condition was another factor that influenced the nickel released from stressed wires. For bending conditions, the concentration of nickel release from NiTi wires immersed in the artificial saliva solution at pH 2 was significantly higher than at pH 5.3 for the same immersion period ( P <0.005). After soaking for 1 day, as listed in Table I , the normalized concentrations of nickel from bending Nitinol wires were 30.1 μg per square centimeter in artificial saliva solution at pH 2 and 20.0 μg per square centimeter in pH 5.3. For the stressed Sentalloy NiTi wires, the concentration of nickel release in the pH 2 artificial saliva solution was 6.9 times greater compared with pH 5.3 solution after 1 day of soaking. At pH 5.3, the nickel ion release for stressed Nitinol NiTi wires with different immersion periods can be generally ranked in the following increasing order: days 0 to 1, 0 to 3, and 0 to 7; and days 0 to 14. Higher levels of nickel ion release from stressed Nitinol wires were observed after 14 days of immersion, whereas the differences in ion release of days 0 to 1, 0 to 3, and 0 to 7 were not statistically significant. At pH 2, the effect of immersion period on ion release of stressed Nitinol wires can be ranked in the following increasing order: days 0 to 1, 0 to 3, and 0 to 7; and days 0 to 14. For the stressed Sentalloy NiTi wires at pH 5.3, the nickel ion release for different immersion periods can be ranked in the following increasing order: day 0 to 1; days 0 to 3 and 0 to 7; and days 0 to 14. At pH 2, the nickel ion release from stressed Sentalloy wires with different immersion periods can be generally ranked in the following increasing order: days 0 to 1 and 0 to 3; days 0 to 7; and days 0 to 14.
| Time of immersion (d) | pH 2 | pH 5.3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Nitinol | Sentalloy | Nitinol | Sentalloy | |||||
| Unstressed | Stressed | Unstressed | Stressed | Unstressed | Stressed | Unstressed | Stressed | |
| 0-1 | Nil | 36.8 ± 5.9a | Nil | 30.1 ± 5.1a | Nil | 20.0 ± 6.1a | Nil | 4.3 ± 1.6a |
| 0-3 | Nil | 39.7 ± 7.2a | Nil | 27.7 ± 5.1a | Nil | 18.7 ± 5.1a | Nil | 7.2 ± 1.4b |
| 0-7 | Nil | 51.2 ± 8.5a | Nil | 37.8 ± 6.9b | Nil | 25.8 ± 5.1a | Nil | 7.2 ± 1.6b |
| 0-14 | Nil | 90.9 ± 13.8b | Nil | 68.3 ± 10.7c | Nil | 54.2 ± 10.4b | Nil | 12.6 ± 3.4c |
As shown in Figure 2 , the typical surface morphologies of both as-received NiTi wires were observed by SEM. Compared with Sentalloy NiTi wires, Nitinol NiTi wires displayed the longitudinal texture produced during the manufacturing process, and both specimens showed local defects at higher magnifications. After soaking in artificial saliva solution for 14 days, the surface of NiTi wires was observed by SEM ( Fig 3 ). For both pH 2 and 5.3 conditions, the absence of visual corrosion signs on the surfaces of both unstressed and stressed NiTi wires indicates that no obvious degradation occurred. Before (as-received condition) and after soaking in artificial saliva, the surfaces of Nitinol and Sentalloy NiTi wires were observed by SPM ( Figs 4 and 5 ). Compared with as-received NiTi wires, the unstressed and stressed specimens showed smooth surfaces after 14 days of immersion in artificial saliva solution at both pH 2 and 5.3. However, at both pH 2 and pH 5.3 in artificial saliva, the stressed Nitinol NiTi wires showed more irregular surfaces than unstressed wires on the tensile side of specimens bent at 3 points. Similarly, a smooth surface was also observed in the central zone of stressed Sentalloy wires at both pH 2 and 5.3 solutions compared with as-received specimen ( Fig 5 ). Among the 4 groups of Sentalloy NiTi wires, the stressed specimens soaked in pH 2 solution was the only group that showed irregular surfaces by SPM observation.
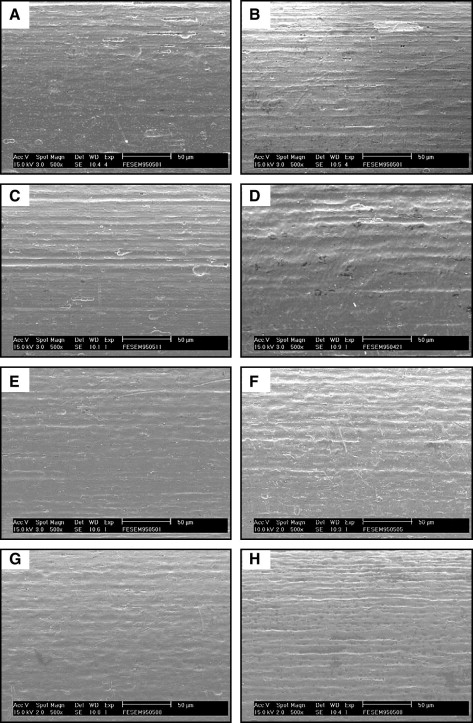
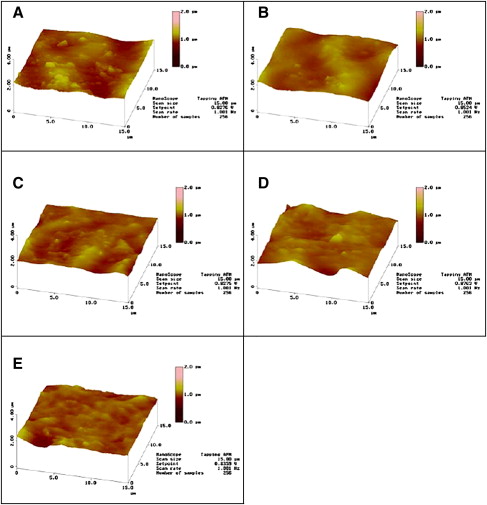
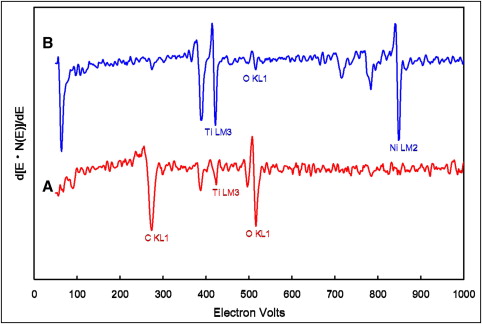
Figure 6 shows the AES spectra of unstressed Nitinol NiTi wires after immersion in artificial saliva solution at pH 5.3 for 14 days. As shown in Figure 6 , A , the AES analysis indicates that the top surface of NiTi wires is mostly constituted of oxygen and carbon with less titanium. The carbon could be attributed to surface contamination from the atmosphere. After 370 seconds of sputtering, the AES spectrum mainly contained titanium and nickel with less oxygen ( Fig 6 , B ). The AES depth-profile analysis of the surface composition of the unstressed Nitinol NiTi wires after 14 days of immersion in pH 5.3 solution is shown in Figure 7 . The result shows that the outermost surfaces of the wires were mainly composed of titanium and oxygen, and the nickel content increased gradually with depth. The oxygen concentration diminished gradually with depth on unstressed Nitinol NiTi wires in pH 5.3 artificial saliva, reaching a relative minimum after 420 seconds of sputtering ( Fig 7 ). For stressed Nitinol NiTi wires soaked in pH 5.3 solution for 14 days, the signal decreased to a relative minimum after 300 seconds in the tensile side of specimens bent at 3 points. After immersion in pH 5.3 artificial saliva, the AES depth-profile chemical compositions of Sentalloy NiTi wires were similar to those of the Nitinol specimens. Thicker oxide films were also measured on the surfaces of the unstressed Sentalloy wires compared with stressed specimens at 14-day immersion periods. Similar results were observed for NiTi wires in pH 2 artificial saliva. Titanium and oxygen were the major elements in the outermost surfaces of the tested specimens in pH 2 solution. As shown in Table II , a thicker oxide film was formed on the surface of unstressed wires compared with the stressed specimens from the same manufacturer in the same pH condition and immersion period.