Fig. 2.1
Scanning electron microscope (SEM) micrographs of the dentin structure. A – Peritubular dentin. B – Dentinal tubules. C – Collagen fibrils
The primary function of the human dentition is the preparation and processing of food through a biomechanical process of biting and chewing. This process is based on the transfer of masticatory forces mediated through the teeth (Versluis and Versluis-Tantbirojn 2011). Although the primary function of the dentin is mechanical, it has only been in the last few decades that its mechanical properties have begun to be understood in terms of its hierarchical microstructure (Kinney et al. 2003; Nalla et al. 2003). Understanding the mechanical behavior of the dentin and the detailed relations to the dentinal structure provides insight into the design strategies to recover tooth functions and helps to improve dental restoration techniques (Eltit et al. 2013).
The ultimate tensile strength of the dentin varies widely and is dependent on intra-tooth location and depth (Giannini et al. 2004). Soares et al. (2010) showed higher ultimate tensile strengths when a tensile force was directed perpendicular to tubule orientation. This may have been a result of the dentinal tubules being surrounded by mineralized collagen fibrils organized layer by layer on planes transverse to the tubules (Eltit et al. 2013).
The tooth is naturally organized to support and transfer stresses and strains generated by masticatory loads. The synergism of the enamel, coronal dentin, and root dentin creates an integrated organ that is capable of supporting high masticatory stresses (Fig. 2.2) (see mechanical properties of the enamel and dentin in Table 2.1). The root dentin is an important component in this structure to integrate the dentition with muscle–bone support. Human root dentin has higher flexural strength and more significant inelastic deformation than the coronal dentin (Eltit et al. 2013). When the tooth crown is structurally compromised by caries or defective restorations, a root canal treatment may be necessary to maintain tooth integrity and to provide stability for coronal rehabilitation.
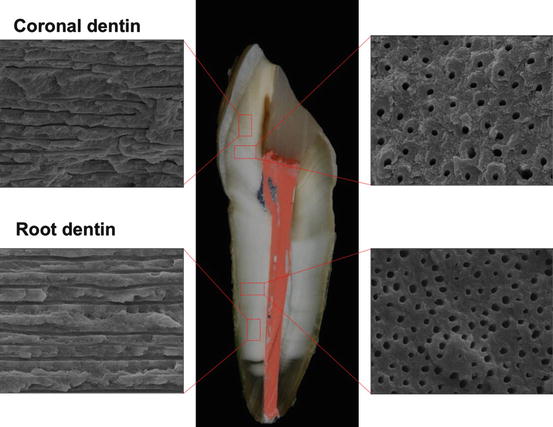

Fig. 2.2
Scanning electron microscope (SEM) micrographs of the structure of the coronal and root dentin perpendicular and parallel to dentin tubules
Table 2.1
Mechanical properties of tooth structure (mean and standard deviation)
|
Tooth structure
|
Ultimate tensile strength – MPaa
|
Compressive strengthb – MPa
|
Elastic modulus – GPa
|
|
|---|---|---|---|---|
|
Parallel to prism/tubule direction
|
Perpendicular to prism/tubule direction
|
|||
|
Enamel
|
40.1 (12.4)
|
17.6 (5.7)
|
261 (41.4)
|
87.5 (2.1)c
|
|
Coronal dentin
|
54.2 (19.5)
|
86.5 (20.2)
|
282.6 (34.5)
|
18.0 (1.5)d
|
|
Root dentin
|
49.8 (13.9)
|
64.7 (14.7)
|
233.5 (26.7)
|
21.5 (2.1)d
|
2.2 Factors That Predispose Endodontically Treated Teeth to Fracture
The causes of tooth fracture have been associated with occurrence of sudden impact trauma resulting from falls, fights, motor vehicle accidents, epileptic fits, and laryngoscope misuse, and with fatigue failure of tooth structure resulting from repeated stress overloading (Tang et al. 2010). When loads are applied to a structure it deforms (strain) and stresses are generated. This is how a tooth performs its structural function. But if such stresses become excessive and exceed the strength of the tooth, structural failure may occur (Soares et al. 2008).
Endodontic treatment is a common clinical procedure, usually provided with the objective of retaining teeth in which the pulp has become irreversibly infected or necrotic (Driscoll et al. 2002). Endodontic procedures include the removal of pulp tissue and the heavily infected dentin surrounding the pulp. These procedures involve mechanical and chemical events that may interfere with the natural stress–strain distribution in the tooth structure, increasing the risk of failure.
The fracture of endodontically treated teeth is a common clinical failure, which could require extraction. A factor that may predispose such teeth to fracture has been identified as changes in the mechanical properties of the dentin (Fig. 2.3). Dentinal collagen makes a considerable contribution to the mechanical properties of the dentin (Grigoratos et al. 2001). Changes in these collagen fibril cross-links may contribute to the so-called brittleness of the pulpless teeth (Soares et al. 2007). More immature and fewer mature cross-links in endodontically treated teeth might account for a decrease in tensile strength (Gutmann 1992). Loss of pulp vitality also influences moisture content of the dentin. Additionally, iatrogenic factors associated with various operative procedures may contribute to the fracture of the endodontically treated teeth, although most of these risks are controllable (Tang et al. 2010).
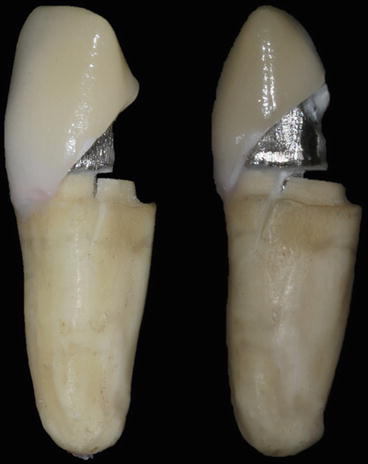

Fig. 2.3
Fracture of an endodontically treated tooth demonstrating fragility of the root dentin
2.2.1 Immature Teeth with Incomplete Root Formation
Immature teeth with diseased or necrotic pulps generally exhibit arrested root development and open apices. After endodontic treatment, immature teeth are severely weakened because of wide, flared canal spaces and thin dentin walls (Brito-Junior et al. 2014). Apexification and root rehabilitation with a relined glass fiber post (see Chap. 5 (Fig. 5.4)) might restore the reduced mechanical integrity resulting from incomplete root formation (Tang et al. 2010; Brito-Junior et al. 2014). Furthermore, finite element analysis demonstrated that endodontically treated teeth without fiber post had higher stress concentrations in the root dentin than those restored with resin composite or other restorative techniques that used fiber posts (Fig. 2.4). The restorations with posts exhibited higher fracture resistance (Brito-Junior et al. 2014).
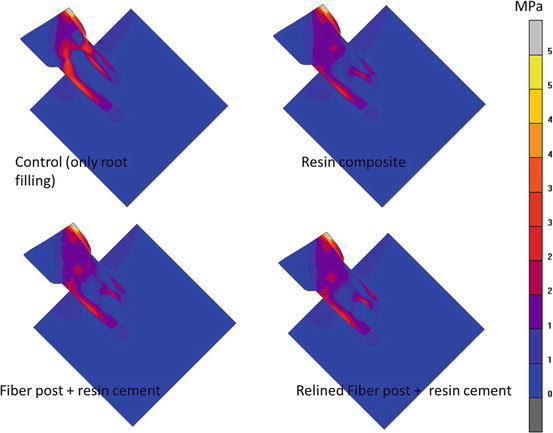

Fig. 2.4
Von Mises stress distributions in the tooth with incomplete root formation restored with different restorative options (Figure adapted from Brito-Junior et al. (2014)). Von Mises stress is an equivalent stress that shows the general stress state of all stress components
2.2.2 Endodontic Access Preparation
The actual effect of the endodontic access preparation is controversial. Some studies have shown that the loss of tooth structure from endodontic access preparation might increase the occurrence of fractures (Zhi-Yue and Yu-Xing 2003). Other authors showed that a conservative root canal access resulted in less tooth deformation (Tang et al. 2010).
There is a consensus that tooth deformation increases progressively after root canal preparation and obturation, in particular after post space preparations (Tang et al. 2010).
2.2.3 Root Canal Preparation: Stress Distribution Generated in Root Dentin During Root Canal Shaping and Effect on Vertical Root Fracture
Endodontic treatment is a predictable therapy with a success rate up to 97 % (Salehrabi and Rotstein 2004). However, many completed cases may still be lost over the patient’s lifetime. The most common reasons for catastrophic failure of endodontically treated teeth [vertical root fracture (VRF)] are weakening of the residual tooth structure by caries and overpreparation and the post system used during the rehabilitation (Fuss et al. 2001; Tsesis et al. 2010). VRF is one of the most disappointing complications of root canal treatment, which may result in a tooth extraction (Tamse 2006). VRF of endodontically treated teeth is a clinical problem of increasing significance.
VRF is usually diagnosed a few years after the endodontic and restorative treatments are completed (Vire 1991; Fuss et al. 2001). Root canal preparation procedures have been reported to increase the risk of root fracture and crack formation by reducing root dentin integrity or creating defects inside root canals that may act as stress concentration or crack initiation sites (Schafer and Lau 1999; Schafer et al. 2004; Adorno et al. 2009; Kim et al. 2010).
Many nickel–titanium (NiTi) rotary instruments have been developed and introduced in the last decades. These instruments have brought convenience and efficiency to root canal shaping, which reduces procedural errors (Schafer and Lau 1999; Schafer et al. 2004). Most clinicians prefer these systems because of their advantages, such as improved cutting efficiency and time saving (Schafer and Lau 1999; Schafer et al. 2004). Nevertheless, some functions of NiTi rotary systems such as their cleaning ability, increased stresses accumulated in the root dentin, and the inability to adequately prepare oval canals, are still controversial. File design is likely to affect the shaping forces on the dentin during instrumentation (Lam et al. 2005; Kim et al. 2008). During instrumentation a root canal is cut and enlarged by the contact between instruments and dentin walls. The numerous momentary contacts between instrument and canal wall create stress concentrations in the dentin and may induce dentinal defects and microcracks or craze lines (Blum et al. 1999; Kim et al. 2008, 2010). Adorno et al. (2009) reported that root canal preparation alone significantly weakened roots, especially when the working length is longer than optimal, and may create apical root cracks. Bier et al. (2009) showed dentinal damage, such as craze lines and partial cracks, in teeth that were prepared with several brands of NiTi rotary instruments, whereas no defect was observed with hand files (Bier et al. 2009). This type of defects are associated with increased susceptibility to VRF because functional loads or stresses from the repeated occlusal forces after restoration can be amplified at the tip of those defects and initiate or propagate into VRF (Tamse et al. 1999; Kim et al. 2010).
Stresses in the root dentin during instrumentation by various NiTi instruments have been studied using finite element analysis (FEA). Kim et al. (2010) investigated the potential relationship between NiTi instrument design and the incidence of VRF and crack formation. This study compared stresses generated in the apical root dentin during rotary instrumentation in a curved canal with NiTi files featuring different cross sections and shaft geometries. It was shown that during root canal instrumentation, under certain clinical conditions (e.g., in a severely curved canal), file design (with a big taper and/or stiff characteristics) might generate stresses that exceed the tensile strength of the dentin (Fig. 2.5).
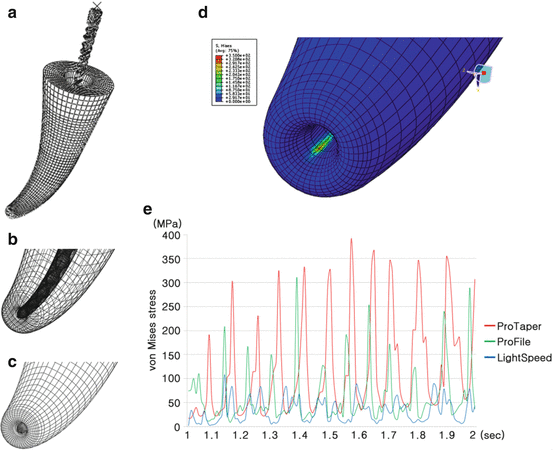

Fig. 2.5
Finite element analysis evaluating stress concentrations during instrumentation. (a–c) Finite element models of the root canal and instrumentation. (d) Representative figure of von Mises stress concentration at the apical dentin during simulated shaping rotation. (e) Graph showing cyclic stress profile during simulated shaping in a node of the model where the highest von Mises stress was generated (Kim et al. 2010)
On the other hand, some NiTi instrument systems for coronal canal flaring, which are used in early root canal preparations, were reported to have lower rates of crack formation than those found with Gates-Glidden drills (Arslan et al. 2014).
Recently introduced reciprocating NiTi instrument systems use a back-and-forth rotational movement instead of continuous rotation (Yared 2008). Many articles have since been published about reciprocating NiTi instruments, which are used in a single file technique for clinical convenience and efficiency (Kim et al. 2012; Shin et al. 2014). The conclusions regarding apical crack formation in many of these articles are contradictory (Bürklein et al. 2013; Abou El Nasr and Abd El Kader 2014; De-Deus et al. 2014). Although root canal preparation with either rotary or reciprocating instruments resulted in dentinal defects, reciprocating files resulted in significantly more incomplete dentinal cracks than full-sequence rotary systems at the apical level of the canals (Bürklein et al. 2013). On the other hand, ProTaper rotary files (Dentsply Maillefer) were reported to have significantly more microcracks than ProTaper hand files and WaveOne Primary reciprocating files (Dentsply Maillefer). NiTi hand K-files did not produce microcracks at any level inside the root canals (Ashwinkumar et al. 2014). In one article, with regard to the potential risk of root cracks from the file system, WaveOne instruments induced the least amount of cracks and exhibited the greatest resistance to fracture compared with ProTaper F2 files whether used in reciprocating or rotating motions. It was suggested that the alloy from which the instrument was manufactured was a more important factor for the dentin damaging potential than the motion of instrumentation (Ashwinkumar et al. 2014). Other authors found no causal relationship between dentinal microcrack formation and root canal preparation procedures with the reciprocating systems (De-Deus et al. 2014).
In another recent development, the design of a self-adjusting file (SAF) – an instrument without an internal core and with a mesh-like structure – may produce minimal stress concentrations in the apical root dentin during shaping of the curved root canal, which may increase the chance of preservation of root dentin integrity with a reduced risk of dentinal defects and apical root cracking (Yoldas et al. 2012; Kim et al. 2013; Liu et al. 2013).
Comparing primary root canal treatment with retreatment, retreatment procedures may result in more defects than initial treatments (Shemesh et al. 2011). Therefore, it was suggested that when assessing the outcomes of endodontic retreatment, substantial damage to the root canal walls should be considered. Practitioners that are aware of controllable and uncontrollable risks during dental treatments may be able to reduce potential tooth fractures (Tang et al. 2010).
Although the precise effect of each shaping procedure is still debatable, it is well accepted that stress levels during shaping and the susceptibility to apical root cracking after shaping vary with instrument design.
2.2.4 Loss of Dentin During Endodontic Treatment
The removal of the inner dentin negatively influences the stress distribution (Santos-Filho et al. 2014). In some situations, root canals become flared because of the progression of caries associated with endodontic access and overpreparation. The resulting flared root canals have thin root dentin walls, which may be too weak to resist physiologic occlusal loading, thus becoming more susceptible to fatigue and fracture (Coelho et al. 2009; Silva et al. 2011). This fact makes the restorative procedure of these teeth more difficult. Therefore, the rehabilitation of weakened root-filled teeth has been recognized as a challenge. In Chap. 5 (Section 5.4) we will explore in detail a method that uses relined glass fiber posts to create a homogeneous stress distribution to the external dentin surface that is similar to a sound tooth (Santos-Filho et al. 2014; Coelho et al. 2009).
2.3 Modifications Caused by Irrigants, Medicaments, and Root Canal Filling Materials on Mechanical Properties of the Root Dentin
The success of root canal treatment depends on the root canal system being thoroughly cleansed and disinfected. To complement proper cleaning and shaping of the root canal system, filling with a biologically inert and dimensionally stable material is a major objective of root canal treatment (Heulsmann et al. 2005). Substances used during chemical–mechanical preparation may alter the collagen structure, which contributes considerably to the mechanical properties of the dentin (Renovato et al. 2013). Sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA) are common solutions used as endodontic irrigants. Saleh and Ettman (1999) evaluated the effect of several endodontic irrigation solutions on the microhardness of the root canal dentin. The canal portions in the root segments were irrigated with 3 % H2O2, 5 % NaOCl, and 17 % EDTA. The results showed that irrigation with either H2O2/NaOCl or EDTA decreased the microhardness of the root dentin. Irrigation with EDTA lowered dentin hardness compared to H2O2/NaOCl irrigation. These irrigants also changed the mechanical properties of the dentin, such as flexural strength and elastic modulus. Grigoratos et al. (2001) evaluated the effect of NaOCl (3 and 5 %) and saturated calcium hydroxide (Ca(OH)2) solutions on the flexural strength and elastic modulus of the dentin using a three-point bending test. The results showed that NaOCl reduced both elastic modulus and flexural strength, while the saturated Ca(OH)2 reduced the flexural strength but not the elastic modulus of the dentin. Prolonged use of high concentration of EDTA and NaOCl solutions may thus increase the risk for root fracture. Clinicians should be aware that the irrigants should be completely removed from the canal prior to the root-filling procedure.
Gutta-percha is still the most widely used material for root canal filling in endodontic treatment. Materials used during the root-filling therapy must be radiopaque and easily handled, have antimicrobial properties, and provide a stable adhesive seal and reinforcement of the root canal dentin walls (Tang et al. 2010). Although gutta-percha may provide some support, reinforcement of the canal walls requires adhesion of the endodontic material to root canal walls, which has been shown to improve the fracture resistance of endodontically treated teeth (Tang et al. 2010). In the case of endodontic retreated teeth, additional procedures are involved with the removal of filling material and contaminants that can negatively influence the mechanical properties of the root dentin (Guedes et al. 2014). Signs of endodontic treatment failure, including the presence of apical periodontitis and posttreatment symptoms, are important indicators that further intervention is required (Estrela et al. 2009). During root canal retreatment, the radicular and coronal dentin is exposed to gutta-percha solvents. These solvents may modify the chemical composition of the dentin surface and affect its interaction with the restorative and root-filling materials. The use of xylene and orange oil as gutta-percha solvents during root canal retreatment had no significant differences in the bond strength of the fiberglass posts cemented to the radicular dentin. The use of eucalyptol significantly decreased the bond strength of fiberglass posts in the cervical and middle third of the roots, with higher bond strength values in the cervical third than the apical third (Guedes et al. 2014).
2.4 Structural Moisture Content of Endodontic Treated Teeth
In the confined environment of a vital pulp and adjacent dentinal tubules, the presence of free water results in increased dentin viscoelasticity and also facilitates the absorption and distribution of energy before tooth fracture occurs (Tang et al. 2010). On the other hand, the dehydration of the dentin tends to increase its elastic modulus, proportional limit, and especially ultimate strength, by increasing the stiffness and decreasing the plasticity beyond the proportional limit (Tidmarsh 1976). The reduction in tooth structure and the effect of dehydration on the dentinal tubules are widely considered to be the main reasons for increased brittleness associated with endodontic treated teeth (Tidmarsh 1976). The moisture content of the coronal dentin is approximately 13.2 %, but the coronal dentin has twice the number of tubules than radicular dentin per unit area (Gutmann 1992). Presumably, with fewer tubules, the radicular dentin would have lower moisture content. During aging, greater amounts of the peritubular dentin are deposited, decreasing the amount of organic materials that may contain moisture. Physiological transparent dentin is first formed in the apical dentin adjacent to the cementum and extends coronally and toward the root canal with increasing age (Vasiliadis et al. 1983). The physiological transparent root dentin, as distinguished from pathological transparency subjacent to caries, appears to form without trauma or caries lesion as a natural part of aging (Vasiliadis et al. 1983). In addition, the mineral concentration is significantly higher in the transparent dentin and its fracture toughness decreases by 20 % (Kinney et al. 2005; Perdigao 2010; Tang et al. 2010). Endodontic treated teeth have less moisture than vital teeth. The calcified tissues of pulpless teeth had 9 % less moisture than the vital teeth (Helfer et al. 1972; Lee et al. 2004). This reduction in water content after endodontic treatment could result in dentin tissue shrinkage, inducing stresses leading to crack formation, and these cracks could initiate tooth fracture. This may explain a significant decrease in ultimate strength values observed in the endodontic treated teeth (Soares et al. 2007). Reduced shear strength and toughness have also been reported with a simultaneous reduction in tooth stiffness (Carter et al. 1983; Reeh et al. 1989).
2.5 Effect of Different Canal Tapers on Radicular Stress Distributions
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


