Introduction
The aims of this study were to evaluate the effect of an antibacterial monomer-containing self-etching adhesive in reducing enamel demineralization around orthodontic brackets in vivo and to compare it with the conventional adhesive system quantitatively.
Methods
Fourteen orthodontic patients were randomly divided into 2 equal groups; they received brackets fitted to all their teeth, bonded with either Clearfil Protect Bond (Kuraray Medical, Okayama, Japan) (experimental group) or Transbond XT (3M Unitek, Monrovia, Calif) (control group). Block randomization to obtain equal numbers in each group was used. After 30 days, all first premolars were extracted with orthodontic indications and longitudinally sectioned. Demineralization was assessed by cross-sectional microhardness. Determinations were made at the bracket edge cementing limits and at occlusal and cervical points 100 and 200 μm away from the edge. In all of these positions, 6 indentations were made at depths of 10 to 90 μm from the enamel surface. Analysis of variance (ANOVA) and the Tukey post-hoc test were used. The statistical significance level was set at P <0.05.
Results
ANOVA showed statistically significant differences for adhesive type, position, depth, and their interactions ( P <0.05). The multiple comparison test showed that the antibacterial monomer-containing adhesive was significantly more efficient than the conventional adhesive system, reducing enamel demineralization in almost all evaluations ( P <0.05).
Conclusions
The results indicated that using antibacterial monomer-containing adhesive for bonding orthodontic brackets successfully inhibited caries in vivo. This cariostatic effect was localized at the area around the brackets and was significant after 30 days.
Despite the advances in orthodontic materials and treatment mechanics, the placement of fixed appliances is still linked with a high risk of developing white-spot lesions. The prevalence of new decalcifications among orthodontic patients with fixed appliances is reported to range from 13% to 75%. Previous studies have shown that the rate of demineralization in orthodontic patients was higher than those without orthodontic treatment, and teenagers were at higher risk of demineralization than adults. Placement of fixed orthodontic appliances normally causes an increase in oral colonization by Streptococcus mutans , which in turn increases the risk for the development of dental caries.
To inhibit the development of carious lesions in patients with fixed appliances, bacterial plaque around the appliances should be controlled, and a constant level of fluoride should be maintained in the oral cavity. It has been generally accepted that the combined application of fluoride regimens, oral-hygiene instructions, and dietary control can contribute greatly to the inhibition of demineralization during fixed-appliance treatment. These methods, however, rely on patient compliance. Fluoride-releasing bonding materials showed almost no demineralization-inhibiting effect. For that reason, it has been suggested that the combined use of antimicrobials and fluoride enhances the cariostatic effect.
A new antibacterial and fluoride-releasing self-etching adhesive has been developed and introduced in the dental market. Imazato et al 11-14 reported the achievement of an antibacterial adhesive system by incorporation of the new monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB) that has strong bactericidal activity against oral bacteria. Based on the results obtained for this experimental material, a new single-bottled 5% MDPB-containing primer was developed, and this 2-step mild self-etching and fluoride-releasing adhesive system with this primer was commercialized as Clearfil Protect Bond (Kuraray Medical, Okayama, Japan).
The bonding ability of antibacterial monomer-containing adhesive systems have evaluated in vivo, and the cytotoxicity, antibacterial effect, and shear bond strength of brackets or lingual retainer adhesives have been demonstrated by in-vitro studies. However, no studies have been performed to investigate the efficiency of this material on enamel demineralization around orthodontic brackets.
Therefore, the aims of this study were to evaluate the effect of an antibacterial MDPB-containing adhesive in reducing enamel demineralization around orthodontic brackets in vivo and to compare it with conventional adhesive systems quantitatively. In this study, the null hypothesis assumed that the antibacterial monomer-containing adhesive suggested for bracket bonding can significantly reduce the overall amount of demineralization around orthodontic brackets in the mouth.
Material and methods
This study was approved by the Ethical Committee on Research of Gulhane Military Medical Academy, Ankara, Turkey. Fourteen orthodontic patients, 13 to 17 years of age (mean, 14.30 ± 1.65 years), scheduled to have 4 first premolars extracted for orthodontic reasons, were invited to participate and signed a consent form. This study was organized as a parallel group design with 1 group receiving the experimental material and the other serving as the control. A power analysis was established by G∗Power software (version 3.0.10, Franz Faul, Universität Kiel, Kiel, Germany). Based on a 1:1 ratio between groups, a sample size of 14 patients would give more than 80% power to detect significant differences with a 0.40 effect size and at α = 0.05 significance level. The patients were divided into 2 groups of 7 each. Block randomization to obtain equal numbers in each group was used. For group standardization, before starting the procedure, all patients’ teeth were evaluated clinically and radiographically to determine the baseline carries risk. Eight participants (57%) were boys, and 6 (43%) were girls.
In group 1 (Transbond XT, 3M Unitek, Monrovia, Calif; control), there were 4 boys and 3 girls (mean age, 13.85 ± 1.40 years); in group 2 (Clearfil Protect Bond, antibacterial MDPB-containing adhesive), there were 4 boys and 3 girls (mean age, 14.80 ± 1.85 years).
Salivary flow rate and buffer capacity of the patients were recorded. The criteria for including patients were no active caries lesions, normal salivary flow rate (>1.0 mL/min), and buffer capacity (final pH, 6.7-7.7). All patients received a full-mouth cleaning to remove plaque in preparation for bonding. There were no visible signs of caries, fluorosis, or developmental defects in the teeth used. For evaluating the baseline demineralization values of all selected teeth, a portable battery-powered laser fluorescence device, DIAGNOdent Pen (KaVo, Biberach, Germany), was used, and the 2 groups’ scores were low (<13) indicating no demineralization; both were equivalent for caries risk. Orthodontic brackets were bonded with 1 of the following methods.
In group 1 (Transbond XT, control), all teeth were etched for 15 seconds with 37% ortho-phosphoric acid (3M Dental Products, St Paul, Minn), rinsed with water from a 3-in-1 syringe for 15 seconds, and dried with an oil-free source for 15 seconds. Before bracket placement, Transbond XT primer was applied to the etched surfaces in a thin uniform coat. The primer was cured for 10 seconds. Adhesive paste (Transbond XT) was applied to the bracket base, and the bracket was positioned on the facial surface and pressed firmly into place. The excess adhesive was removed from around the bracket with a scaler.
In group 2 (Clearfil Protect Bond), all teeth were etched similar to group 1 for 15 seconds. The self-etching primer containing the antibacterial monomer Clearfil Protect Bond was applied to the etched surfaces for 20 seconds and sprayed with a mild air stream to evaporate the solvent. Then Clearfil Protect Bond was applied, gently air dried, and light cured for 10 seconds. After these steps, a thin layer of the Transbond XT adhesive paste was also applied to the base of the bracket and immediately pressed into the adhesive on the tooth surface.
Stainless steel orthodontic premolar brackets (Dyna-Lok series, 3M Unitek) were bonded by a standard protocol. A light-emitting diode light unit (Elipar Freelight 2, 3M ESPE, St Paul, Minn) was used for curing the specimens for 20 seconds.
For the testing procedure, 28 brackets were cemented for each group (14 maxillary and 14 mandibular first premolars in both groups). After 30 days, the brackets were removed; the teeth were extracted and stored in a refrigerator in flasks containing gauze dampened with 2% formaldehyde, pH 7.0, until the analysis. Demineralization in the enamel around the brackets was evaluated by the cross-sectional microhardness method according to the literature. During the experimental period and 3 weeks before it started, the subjects brushed their teeth with a nonfluoridated dentifrice, but they drank fluoridated water. They received no instructions regarding oral hygiene, kept their usual habits, and were instructed not to use any antibacterial substance.
For the cross-sectional microhardness analysis, 1 operator (S.O.), who was blinded from the group allocation, carried out the microhardness analysis. The roots were removed 2 mm apical to the cementoenamel junction, and the crowns were hemi-sectioned vertically into mesial and distal halves with a 15 HC (large) wafering blade on a low-speed saw (Isomet, Buehler, Lake Bluff, Ill) directly through the slot of the bracket, leaving a gingival portion and an incisal portion. The teeth were embedded in self-curing epoxy-resin (EpoKwick, Buehler), leaving the cut face exposed. The half-crown sections were polished with 3 grades of abrasive paper disks (320, 600, and 1200 grit); final polishing was done with a 1-μm diamond-spray and a polishing-cloth disk (Buehler). A microhardness tester (HMV-700, Shimadzu, Kyoto, Japan) under a 2N load was used for the microhardness analysis.
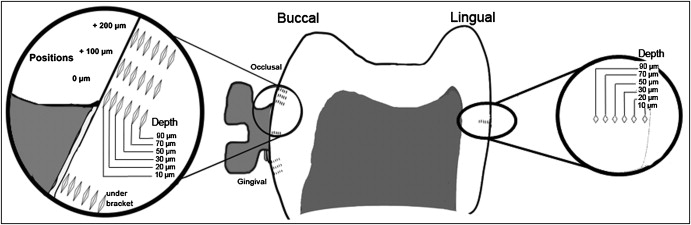
Forty-eight indentations were made in each half crown ( Fig ) from 8 positions and 6 depths according to the definitions of Pascotto et al. On the buccal surface, indentations were made under the bracket. In the occlusal and cervical regions, indentations were made at the edge (0) of the bracket and at 100 and 200 μm from it. Indentations were also made in the middle third of the lingual surface of each half crown, as another control. In all these positions, 5 indentations were made at 10, 20, 30, 50, 70, and 90 μm from the external surface of the enamel. The values of microhardness numbers found in the 2 half crowns were averaged.
Statistical analysis
Data analyses were performed by using the Statistical Package for Social Sciences (version 13.0, SPSS, Chicago, Ill) and Excel 2007 (Microsoft, Redmond, Wash). The Shapiro-Wilks normality test and the Levene variance homogeneity test were applied to the microhardness data. The data showed normal distribution, and there was homogeneity of variances between the groups.
Analysis of variance (ANOVA) was used to evaluate the effect of adhesive types (Transbond XT and Clearfil Protect Bond), depths from the enamel surface (10, 20, 30, 50, 70, and 90 μm), positions (under the bracket, on the buccal surfaces in the occlusal and cervical regions at 0, 100, and 200 μm from the brackets, and on the lingual surfaces), and their interactions. For multiple comparisons, the Tukey post-hoc test was used. Significance was predetermined at P <0.05.
For evaluating the intraobserver and interobserver agreement, the microhardness measurements were done by 2 investigators (S.O. and A.E.K.) using the same instrument at 2 separate times, and Cohen kappa scores were determined.
Results
The kappa scores for the assessment of intraexaminer and interexaminer agreement were higher than 0.75, implying substantial agreement between the observers.
ANOVA of the data showed statistically significant effects for the factors adhesive type, position, and depth, and for the interactions adhesive type ∗ depth, adhesive type∗position, position∗depth, and adhesive type ∗ depth ∗ position ( P <0.05) ( Table I ).
| Source | Sum of squares | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| Corrected model | 2548406.339 † | 95 | 26825.330 | 398212 | 0.000 ∗ |
| Intercept | 264967126.035 | 1 | 264967126.035 | 3933340 | 0.000 ∗ |
| Adhesive type | 131620.316 | 1 | 131620.316 | 1953 | 0.000 ∗ |
| Position | 276830.007 | 7 | 39547.144 | 587063 | 0.000 ∗ |
| Depth | 1348980.974 | 5 | 269796.195 | 4005 | 0.000 ∗ |
| Adhesive type ∗ position | 205859.368 | 7 | 29408.481 | 436558 | 0.000 ∗ |
| Adhesive type ∗ depth | 131462.640 | 5 | 26292.528 | 390303 | 0.000 ∗ |
| Position ∗ depth | 276943.914 | 35 | 7912.683 | 117461 | 0.000 ∗ |
| Adhesive type ∗ position ∗ depth | 176709.121 | 35 | 5048.832 | 74948 | 0.000 ∗ |
Descriptive statistics and multiple comparisons of microhardness for antibacterial monomer-containing and conventional adhesive systems at different depths from the enamel surface are presented in Table II . The interaction between adhesive type and depth showed significant differences at depths of 10, 20, and 30 μm from the enamel surface. Less lesion depth was found in enamel around the brackets bonded with antibacterial monomer-containing adhesive in comparison with the conventional system.
| Interaction of adhesive type ∗ depth | Transbond XT | Clearfil Protect Bond | Multiple comparisons ∗ | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 10 μm | 260.968 | 7.717 | 297.996 | 11.577 | – |
| 20 μm | 281.365 | 9.512 | 307.903 | 9.561 | – |
| 30 μm | 301.366 | 10.959 | 317.588 | 9.161 | – |
| 50 μm | 322.222 | 9.536 | 325.156 | 7.111 | NS |
| 70 μm | 328.802 | 8.484 | 330.161 | 7.915 | NS |
| 90 μm | 347.080 | 8.414 | 346.969 | 6.928 | NS |
Multiple comparisons of microhardness of 2 adhesive types at 8 observation positions under the brackets, and occlusal and cervical to the brackets on the labial and lingual (control) surfaces are given in Table III . The interaction adhesive type ∗ position showed statistically significant differences between the materials at the cervical (0 and 100 μm) and occlusal (0 and 100 μm) regions of the bracket ( P <0.05). The greatest mineral loss (lowest microhardness) was observed at the 0 μm cervical region (270.132 ± 24.956) for the control group.
| Interaction of adhesive type ∗ position | Transbond XT | Clearfil Protect Bond | Multiple comparisons | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Occlusal 200 μm | 316.603 | 20.556 | 318.967 | 19.136 | NS |
| Occlusal 100 μm | 310.785 | 25.655 | 323.823 | 15.072 | ∗ |
| Occlusal 0 μm | 282.736 | 23.207 | 320.648 | 19.198 | ∗ |
| Under bracket | 324.376 | 17.083 | 322.800 | 15.906 | NS |
| Cervical 0 μm | 270.132 | 24.956 | 317.032 | 21.554 | ∗ |
| Cervical 100 μm | 307.598 | 21.040 | 321.779 | 17.370 | ∗ |
| Cervical 200 μm | 318.670 | 20.741 | 317.451 | 19.099 | NS |
| Lingual | 324.836 | 15.766 | 325.199 | 14.683 | NS |
The Tukey post-hoc test was applied to the triple interaction (adhesive type ∗ depth ∗ position), and the results are shown in Table IV . These results showed statistically significant differences at 4 positions (cervical and occlusal regions 0 and 100 μm from the bracket edge) evaluated on the buccal surfaces at 10, 20, and 30 μm depths from the surface of the enamel. There was no significant difference between the groups in the hardness observed under the bracket and at the lingual surface of the teeth.



