Introduction
The purposes of this study were to differentiate embryonic limb bud cells into cartilage, characterize the nodules produced, and determine their ability to heal a mouse skull defect.
Methods
Aggregated mouse limb bud cells (E12-E12.5), cultured in a bioreactor for 3 weeks, were analyzed by histology or implanted in 6 skull defects. Six controls had no implants. The mice were scanned with microcomputed tomography weekly. At 2 and 4 weeks, a mouse from each group was killed, and the defect region was prepared for histology.
Results
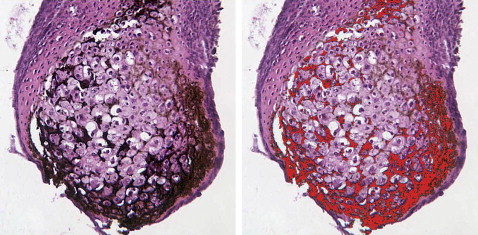
Chondrocytes in nodules were mainly hypertrophic. About 90% of the nodules mineralized. BrdU staining showed dividing cells in the perichondrium. Microcomputed tomography scans showed increasing mineral in implanted nodules that completely filled the defect by 6 weeks; defects in the control mice were not healed by then. At 2 and 4 weeks, the control skull sections showed only a thin bony layer over the defect. At 2 weeks, bone and cartilage filled the defects with implants, and the implants were well integrated with the adjacent cortical bone. At 4 weeks, the implant had turned almost entirely into bone.
Conclusions
Cartilage differentiated in the bioreactor and facilitated healing when implanted into a defect. Engineering cartilage to replace bone is an alternative to current methods of bone grafting.
Editor’s summary
Reasons for autogenous bone grafting are now almost too numerous to mention. In the final stages of treating cleft lip and palate patients, grafts might be needed in the cleft area before the placement of implants, or to close an oronasal fistula, for proper bony support next to the cleft, or even for increased support for the alar base. In implant dentistry, ridge augmentation is frequently needed to improve the functional and esthetic outcome of the implant. In other patients, a deficient alveolar ridge can affect phonetics and esthetics. I could go on, but why? You would think this study was as important as stem cell research. Well, that’s what it’s all about. This study confirms previous results showing that engineered bone-forming cartilage is an excellent way to heal even membranous bone.
These authors investigated using in-vitro-differentiated cartilage—the bone-forming kind—to facilitate the healing of skull defects in mice. The aim was to assess histologically the stage and amount of cartilage produced after 3 weeks of culture. This was evaluated with microcomputed tomography scanning and histology, noting the ability of cartilaginous spheroids used to heal a defect in a membranous bone. The authors concluded that engineering cartilage could be an alternative method to repair the defect of a membranous bone.
The details of the study are published online and will be of special interest to those with a background in histology. Noted the authors, “Human bone-marrow stem cells will be used next to form cartilage in culture, so that we can proceed toward the goal of providing implants to patients constructed from their own stem cells.” With this type of basic research in our universities, the future looks a little brighter.