Fig. 6.1
Flapless surgery for implant placement using navigation guides. Both the depth and the position of the implant osteotomy are determined by the guide
6.2.2 Local Anesthetic Injection
The IAN or LN can be injured by needle contact secondary to the injection of a local anesthetic into the pterygomandibular space [27, 28] or the MN when injecting in the area of the mental foramen. Although the exact pathophysiology of this injury remains unknown, there are three possible causes: (1) direct intraneural injection with mechanical injury to the nerve (i.e., severance of axons, partial or total, scar tissue or neuroma formation, Wallerian degeneration), (2) interruption of vessels of the mesoneurium with peri- and intraneural hemorrhage and secondary scar formation, and (3) chemical toxicity of the anesthetic solution, or from a contaminant (sterilizing solution in a storage container) that is able to enter into a leaky anesthetic cartridge [13]. Regardless of the cause, it is recommended that aspiration be performed prior to all local anesthetic injections. If there is a bloody aspirate, or the patient complains of a paresthesia (typically, an “electric shock-like” sensation), the needle is withdrawn a few millimeters and aspiration is repeated. If there is now no bloody aspirate, it can be assumed that the needle tip is no longer in contact with a blood vessel or nerve, and the injection is completed. A note of such an occurrence should be routinely entered in the patient’s chart. This technique may prevent direct injection into a vascular space, but does not necessarily prevent deposition of the anesthetic within the epineurium (the diameter of the IAN is four to five times greater than the associated inferior alveolar artery or vein). Nerve injury secondary to local anesthetic injection, although uncommon, has a reported incidence of 1:26,762 to 1:160,571. It can be difficult to differentiate from injury related to the placement of the dental implants, especially if sedation or general anesthesia was used and, therefore, the patient was unable to report a paresthesia at the time of the injection(s). Without obvious clinical or radiographic signs of injury to the nerve from the dental implant procedure itself, the possibility of needle injection injury cannot be eliminated. Unfortunately, a small number of patients who have suffered an injection-related injury can be misdiagnosed with injury related to the dental implant surgery and subsequently undergo either inappropriate removal of the implant or fruitless exploratory surgical procedures that reveal no visible nerve injury at the implant location.
6.2.3 Osteotomy Preparation
Injury to the IAN as a consequence of bone preparation or implant placement can be due to errors in radiographic planning, drilling, or direct contact of the implant with the nerve. Drilling injuries to the IAN can be difficult to diagnose. Despite correct position of the implant vis-a-vis the IAC on the postoperative radiograph appearance of the implant, osseous preparation with the drill may have been performed beyond the planned implant depth causing injury to the nerve (Fig. 6.2). In addition to the possibility that one of the implant drills entered the IAC and injured the IAN, it is also possible that the drill caused vascular trauma to the inferior alveolar artery (IAA) or inferior alveolar vein (IAV) and resulted in intra-canal bleeding. This bleeding may be noted during the osteotomy preparation by visualization of oozing that is more significant than normal marrow oozing. Once the implant is placed, the bleeding is tamponaded with resultant pressure placed upon the IAN, resulting in paresthesia, and even dysesthesia. This error can be prevented by measurement from correctly calibrated radiographs of the distance from the alveolar ridge crest to the superior aspect of the IAC, the use of drilling equipment with predetermined depth stops, and careful technique to prevent drilling beyond the planned depth. Irrigation with adequate coolant to dispel heat generated by bone drilling may also prevent a thermal injury in the absence of direct contact with the nerve. Frequent intraoperative reverification of the drill dimensions (diameter and length) is also helpful.
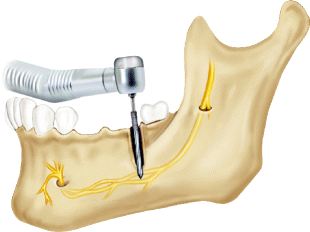

Fig. 6.2
Diagram of a direct injury to the IAN by drilling beyond the planned osteotomy through the superior aspect of the IAC
6.2.4 Direct Implant Placement Injury
In addition to injury caused by drilling, the extent of injury to the IAN due to the implant itself is related to the degree of encroachment of the implant into the IAC or its direct contact with the IAN (Fig. 6.3). Nerve injury due to implant placement may occur despite proper osseous preparation, when the implant is inserted beyond the vertical confines of the prepared bone, compressing or breaching the superior wall of the IAC and forcing bone into the canal (Fig. 6.4a). Alternately, extension of drilling into the IAC may facilitate overinsertion of the implant beyond its intended depth and into the IAC, making direct contact between the implant and the IAN (Fig. 6.4b, c). Finally, delayed osseous healing and remodeling from localized injury can cause excessive bone formation during the healing phases and compromise the IAC cross-sectional diameter resulting in nerve compression (Fig. 6.4d) [8].
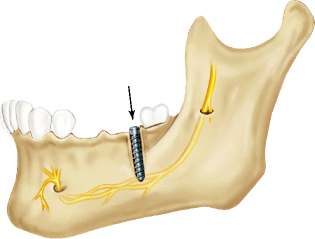
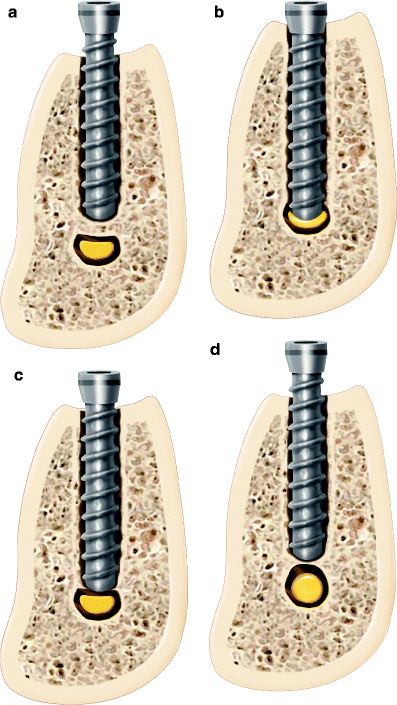

Fig. 6.3
Diagram showing the placement of an implant into the confines of the IAC with increased intra-canal pressure

Fig. 6.4
(a) Collapse of the superior aspect of the IAC due to implant placement beyond the planned osteotomy causing injury to the nerve (compartment syndrome). (b) Direct injury to the IAN by implant contact. (c) Direct injury to the cortical rim of the IAC with deformation of the neurovascular bundle. (d) Remodeling of the IAC cortical rim causing narrowing of canal
6.2.5 Other Causes of Injury
The mental nerve (MN) lies in the mandibular buccal soft tissues and is at risk for iatrogenic injury during a vestibular incision. Recognition of the changing anatomy of the edentulous mandible is particularly helpful in minimizing the risk of injury to the MN. As the patient ages, the alveolar bone in an edentulous area resorbs, and the position of the mental foramen becomes closer to the crest of the alveolar ridge (Fig. 6.5a). In some patients there is actual dehiscence of the IAC, and the IAN and the MN come to lie on the alveolar ridge crest (Fig. 6.5b). Placement of an incision must, therefore, take these anatomic changes into consideration. During the retraction of a mucoperiosteal flap, it is possible to exert continuous, undue pressure on the underlying MN. Gentle soft tissue retraction with frequent brief relaxation of retraction pressure is suggested (Fig. 6.5c).
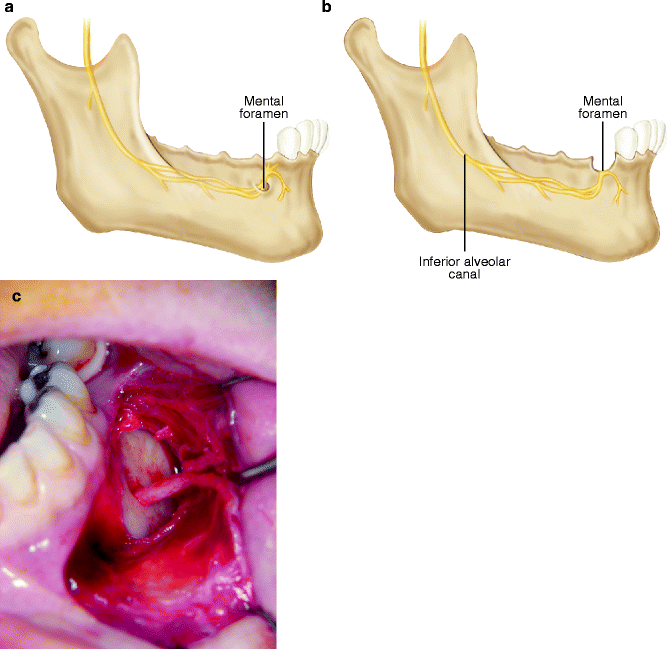

Fig. 6.5
(a) Superior position of the mental foramen due to resorption of the alveolar bone in the partially edentulous mandible. (b) Dehiscence of the IAC, where the IAN and the MN come to lie on the alveolar ridge crest. (c) Exposure of the MN with gentle traction and frequent relaxation minimizes the chance of nerve injury
Less common causes of nerve injury are related to placement of bone grafts (autologous, allogeneic, xenogeneic) during simultaneous implant placement. In cases of complex implant reconstruction, the bone graft material may be placed into the donor site with excessive force, thus severely compressing or even crushing the IAN. It is also possible that particulate bone materials placed in the vicinity of the mental foramen may migrate or become dislodged to impinge upon the MN as it exits the foramen, and this may cause significant scarring around the nerve and resultant paresthesia, including dysesthesia.
6.3 Evaluation of Implant-Related Nerve Injury
6.3.1 Evaluation of Nerve Injuries
Neurosensory disturbances are evaluated and documented in a standard fashion using the Medical Research Council Scale (MRCS) guidelines, as modified for the oral and maxillofacial regions, regardless of the etiology of the sensory nerve injury. The evaluation of nerve injuries is discussed in Chap. 10. Since many of these implant-related injuries result in dysesthesia, specific attention should be directed towards the time frame of the injury and the likelihood that pharmacologic management may be indicated.
6.3.2 Treatment
Timely repair of peripheral nerve injuries has always been the sine qua non for successful recovery of nerve function, especially since Seddon’s extensive experience with treatment of missile injuries to extremities during and following WWII. His comment [31], “(i)f a purely expectant policy is pursued, the most favorable time for operative intervention will always be missed…,” is as pertinent today as it was more than 60 years ago. As in all other causes of nerve injury, treatment of the patient with a dental implant-associated nerve injury is dependent upon the correct diagnosis of the injury and its timely management.
The perioperative administration of supportive medications has been advocated for patients undergoing procedures such as dental implants, mandibular osteotomies, and lower third molar removal that are associated with a significant risk of nerve injury. There is conflict in the literature between those who recommend beginning corticosteroids preoperatively [1] and others who advise waiting postoperatively for several days before initiating administration to allow for edema resolution and tissue perfusion of the medication [32]. Many surgeons routinely administer a single preoperative intravenous dose of a steroid (dexamethasone or hydrocortisone). Whether or not it is beneficial to initiate corticosteroid or anti-inflammatory (NSAID) medications after a nerve injury has occurred is questionable. Previous studies have documented the lack of benefit of corticosteroids administered to reduce cerebral edema in patients who have sustained closed head injuries. That the IAN, in a similar “closed box” situation, confined within the IAC, could benefit from a retroactively administered corticosteroid seems unlikely as well, although that data is conflicting.
An algorithm for the management of nerve injuries from dental implant surgery is shown in Table 6.1. The patient who complains of decreased or painful sensation following placement of dental implants should be requested to return to the office for evaluation. In some patients a nerve injury might have been suspected, if the patient complained of a paresthesia during local anesthetic injection or during the bone drilling preparation for implant placement. In most cases, however, the patient may be under intravenous sedation, and there is typically no indication during the procedure of a nerve injury. It is recommended that the patient be seen as soon as possible and convenient for the patient, preferably within 24 h, or the same day, if painful sensation is the chief complaint, so that adequate pain control can be established and rapport with the patient maintained. The exact nature of the complaint(s) should be ascertained. A general oral exam is performed to assess the healing status of the surgical site. Neurosensory testing (NST) is done to establish an objective baseline determination of the level of sensory dysfunction for further follow-up, as indicated.
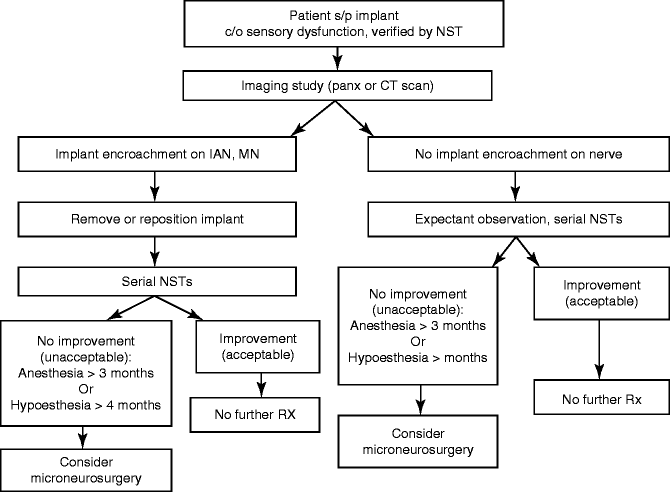
Table 6.1
Algorithm for the management of nerve injuries from dental implant surgery

A panoramic radiograph is obtained to determine the position of the implant(s) in relation to the IAN. If there is no close relationship of the implant and the IAC on the panoramic film, no repositioning or removal of the implant is indicated and should be done. The patient is followed expectantly with frequent repeat NST to assess progress of recovery of sensation. Those patients who go on to acceptable (to the patient) spontaneous recovery require no further active treatment. Patients who fail to regain acceptable sensory function within 3 (anesthesia) or 4 (hypoesthesia ± pain) months are referred to a microneurosurgeon for possible nerve exploration and repair. On the other hand, if there is superimposition of the implant over the IAC on the panoramic film, a CT or CBCT scan is obtained to determine whether this represents an encroachment upon the IAN or IAC, or simply a two-dimensional radiographic overlap that cannot be distinguished on the panoramic radiograph. If the CT demonstrates that the implant is not in contact with the IAC, the implant can be maintained and the patient is followed expectantly with serial NST to determine if spontaneous recovery occurs (see above) (Fig. 6.6a–e).
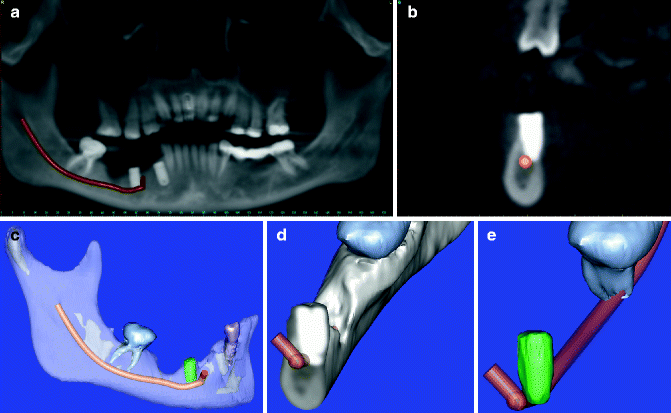

Fig. 6.6
(a) CT-generated panoramic radiograph demonstrating the position of the implant #29 to the IAC in a patient with severe dysesthesia of the IAN following implant placement. (b) Cross-sectional view (coronal) of the same patient demonstrating impingement of the implant on the IAN within the IAC. (c) 3D reconstruction image with transparency of the osseous structures showing the IAC and the implant. (d) 3D reconstruction in cross-section. (e) 3D reconstruction in cross-section with digital removal of the osseous structures
On the contrary, if the implant is in direct contact with the IAC, then the implant should be repositioned immediately (prior to osseointegration) to create at least a 2-mm separation between the apical aspect of the implant and the IAC. If this implant repositioning encroaches unacceptably on the interocclusal clearance, then the implant should be removed and replaced with a shorter implant. This may allow the patient to maintain the implant despite the outcome of nerve injury. If the implant cannot be repositioned without compromising its stability, then it should be removed; consideration could be given towards the use of a shorter implant with a wider diameter to engage the bone for primary implant stability. The patient should be reevaluated with NST within 1 week. If there are signs of neurosensory recovery, no further treatment may be necessary, except for interval NST to document progress to satisfactory return of sensation (“useful sensory function,” or better). The implant can be restored if it has adequate stability and meets acceptable prosthodontic criteria for restoration. It should be remembered that if the implant was close to the IAC, that once the implant is restored and placed into function that neurosensory symptoms may occur during mastication whereby pressure is placed within the closed environment of the IAC. In this case either occlusal adjustment of the implant restoration or removal or “sleeping” the implant may be necessary depending upon the individual patient and clinical symptoms.
If, upon removal or repositioning of the implant, the patient does not show acceptable signs of recovery within 3 (anesthesia) or 4 (hypoesthesia/pain) months by serial NST, microsurgical consultation is indicated. Since the IAN lies within a bony canal, spontaneous recovery might occur due to “guided regeneration” of the nerve provided by the confines of the canal. In such a case, recovery of sensory function should begin (onset of symptoms, responses to NST) within 3 months after nerve injury. Microsurgical consultation can be considered earlier if there is a diagnosis of nerve transection (i.e., by direct visualization at the time of surgery). The so-called 12-week rule for the anesthetic patient has subsequently come to be recognized by many of those surgeons who care for nerve injuries as the standard for timely decision-making for the nerve injury patient who has an unacceptable persistent total loss of sensory function [3–6]. The patient who still has partial, but unacceptable, recovery of sensation at 3 months after nerve injury can be followed at regular (1-month) intervals as long as there is progressive improvement in subjective symptoms and NST at each visit. Once improvement ceases, it will typically not resume at some indeterminate time in the future, and a treatment decision is made at that time, depending upon the level of the sensory deficit according to the NST, the patient’s subjective assessment of his status, and any associated functional impairment.
6.4 Surgical Procedures for IAN Injuries from Dental Implants
A list of microneurosurgical procedures that can provide surgical management of IAN injuries from dental implants is provided in Table 6.2. Figure 6.7 shows various microneurosurgical operations (note: these should include only nerve repairs secondary to dental implant-associated injuries). Although it is beyond the scope of this chapter to discuss all the techniques listed in Table 6.2, in a review of 167 IAN injuries [6], the most commonly performed operation was autogenous (sural or great auricular) nerve grafts (n = 71, 38.2 %) for reconstruction of a nerve gap, followed by internal neurolysis (n = 60, 32.3 %) when the nerve was not discontinuous. The need for reconstruction of a nerve gap was much more frequent with the IAN than that of the LN [4]. This has to do with the greater ease with which the proximal and distal stumps of the LN, contained within soft tissue, are able to be mobilized and brought into approximation for suturing without tension, than is the case with the IAN contained within a bony canal. This certainly has implications for the dental implant patient with a nerve injury, the majority of which are related to the IAN, and not the LN.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


