Figure 4.12 In this CBCT image, osteotomy site #46 shows breach of the roof of the inferior alveolar nerve canal.
F. Extraoral exam
Face and neck
- No asymmetry.
- Skin color of affected area not different from surrounding noninjured area.
- No palpable lymph nodes.
Neurological findings
- Patient has an area of paresthesia (after the surgery) and allodynia (pain to light touch) extraorally in the right mental nerve territory in the mapped area (Figure 4.13).
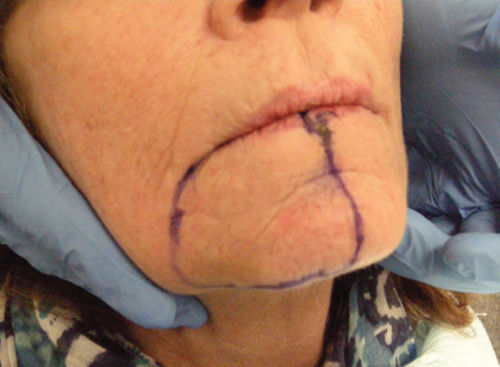
Figure 4.13 Mapped area of sensory changes extraorally at presentation at our center after the surgery.
Temporomandibular joint
- Moderate pain (not familiar) on palpation of the right TMJ.
- Opening click in right TMJ.
- Deviation to the right side with correction toward the midline.
Masticatory muscles
- Mild pain (not familiar) in the right superficial and deep masseter. No pain referral.
G. Intraoral exam
Soft tissues
- Area of paresthesia and allodynia (pain to light touch) intraorally in the buccal gingiva from area 41 to 46 (Figure 4.14).
- There was no color change or swelling in the soft tissues of the injured area.
- The dorsum of the tongue was fissured.
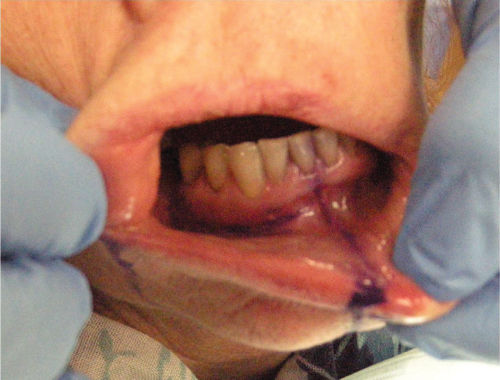
Figure 4.14 Mapped area of sensory changes intraorally.
Hard tissues
- Tooth 46 is missing. All other teeth in the mouth are present, with the exception of third molars.
- Teeth 34, 35, and 36 have crowns.
- Teeth 11, 12, 21, and 22 have veneers.
- Alveolar bone in the area of 46 has a bony depression, where the implant had been placed and removed.
H. Additional Examination and Findings
Neurosensory testing
- A cotton swab was first used to grossly delineate areas of pain and paresthesia. Within the delineated area, two additional tests were performed:
- (1) Mechanical test with Von Frey monofilaments:
- Von Frey monofilaments are graded, calibrated nylon monofilaments, which are capable of delivering predetermined amounts of force.
- There was hypoesthesia in the delineated area compared with the contralateral side.
- (2) Electrical test with Neurometer NervScan NS3000:
- This device can deliver electrical stimuli at different frequencies, which are hypothesized to stimulate different nerve fibers.
- Expressing the electrical detection thresholds as the ratio between the injured and contralateral control side reduces any inconsistency with this method.
- This test showed hyposensitivity in the right mental nerve distribution compared with the contralateral side (Table 4.1).
Table 4.1 Detection thresholds of the affected (right) and unaffected dermatomes to electrical stimuli using the Neurometer
| Frequency used | Right mental (mA) | Left mental (mA) | Ratio (right/left) |
| 5 Hz (C fibers) | 50 | 20 | 2.5 |
| 250 Hz (Aδ fibers) | 90 | 30 | 3 |
| 2000 Hz (Aβ fibers) | 380 | 220 | 1.7 |
mA: milliamperes.
I. Diagnosis
ICHD-3 beta
- Painful post-traumatic trigeminal neuropathy (PTTN).
J. Case Assessment
- The patient’s symptoms and signs fit the diagnostic criteria for PTTN. She had unilateral facial pain located in the distribution of the right inferior alveolar nerve that developed immediately after the implant placement in the same distribution. She also had both a positive (allodynia) and a negative sign (hypoesthesia) of trigeminal nerve dysfunction.
- Localized sensory changes and persistent pain are indicative for PTTN. If left untreated, over time these changes may become persistent. It is important to evaluate these patients early (within days) after the initial injury to:
- rapidly assess the degree of injury;
- determine the possibility of nerve repair;
- establish the early management strategy.
- Imaging is essential to determine the degree of nerve injury, the presence of a “compressing agent” (e.g., an implant), or any pathology (e.g., sequestrum).
- Neurosensory testing is also needed to localize and quantify nerve function.
- Discussion with the patient on management and prognosis.
K. Evidence-based Treatment Plan including Aims
Aims of treatment
- Reduce pain.
- Reduce extent (area) and severity of sensory changes.
Treatment plan
Pharmacologic management
- The patient was prescibed pregabalin 75 mg, three times a day, and duloxetine 20 mg, twice a day. At the same time she was titrated down from the oxycodone–paracetamol combination she was taking (Attal et al., 2010; Finnerup et al., 2015).
Psychosocial management
- The patient was referred for psychological counselling.
L. Prognosis and Discussion
- This case demonstrates how dental implant placement with nerve injury may result in neuropathic complications. In this case example, the patient had both sensory changes and persistent pain.
- Evidence suggests that patients who are female, with a history of painful dental procedures, high pain intensity around the area that is subsequently treated, and with comorbid pain conditions are more likely to suffer from PTTN following nerve injury. Two factors were present in this patient: female sex and fibromyalgia.
- Surgery improved the return of sensation, from complete anesthesia to paresthesia.
- The prognosis in this case is poor, as even after months of pharmacological management the overall pain relief was just under 30% (NRS ratings changed from 7/10 to 5/10).
- Surgery has mostly positive effects on sensory disturbances (Figure 4.15), but less effects on pain. Pharmacotherapy can help with pain management, but has minimal effects on sensory disturbances.
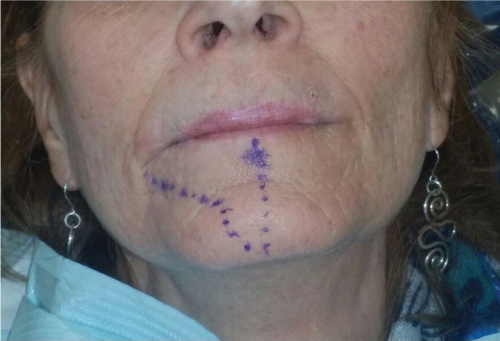
Figure 4.15 Mapped area of injury, 1 month after start of pharmacotherapy, showing shrinkage of the affected area.
Background Information
- Following dental implant surgery, up to 36% of patients may experience transient sensory changes, and up to ∼7% of patients may have permanent sensory changes (unpublished systematic review in our center). The incidence of chronic pain is unclear.
- If surgery is considered, this should be made as early as possible and within the 3-month point if there is no, or minimal, return of sensation or in the presence of dysesthesia. The sensory outcomes are better if the repair is performed within 6 months of the initial injury (Nizam and Ziccardi, 2015).
- The mainstays of neuropathic pain management are the TCA and SNRI antidepressants, and the anti-epileptics. An evidence-based treatment algorithm for painful neuropathy is shown in Figure 4.16.
- PTTN can be associated with a significant psychosocial burden, where patients can display increased depression levels, pain catastrophizing, with lowered coping skills (Smith et al., 2013).
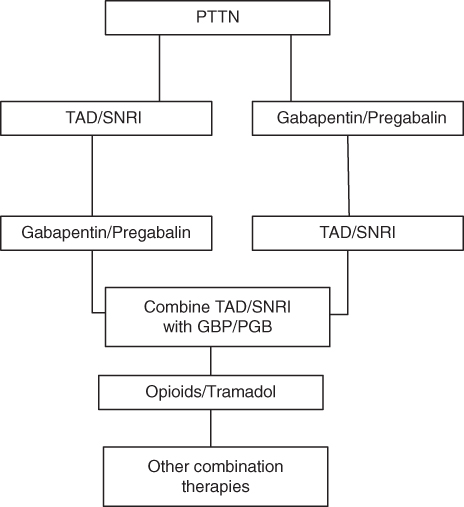
Figure 4.16 Evidence based management of painful neuropathies. Based on the patient’s medical history and patient/physician preference either a TCA/SNRI antidepressant or pregabalin/gabapentin should be initiated. If these fail to offer sufficient relief the alternate drug should be tried, if the medical history permits. Further failure is an indication for a trial of both drugs at the same time. Failure of combination therapy may be an indication for opioid therapy or combined opioid–gabapentin therapy. In areas that are amenable, topical therapy may aid in pain management.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


