Introduction
Pediatric sleep-disordered breathing is a continuum, with primary snoring at one end, and complete upper airway obstruction, hypoxemia, and obstructive hypoventilation at the other. The latter gives rise to obstructive sleep apnea. An important predisposing factor in the development and progression of pediatric sleep-disordered breathing might be craniofacial disharmony. The purpose of this systematic review and meta-analysis was to elucidate the association between craniofacial disharmony and pediatric sleep-disordered breathing.
Methods
Citations to potentially relevant published trials were located by searching PubMed, Embase, Scopus, and the Cochrane Central Register of Controlled Trials. The MetaRegister of controlled trials database was also searched to identify potentially relevant unpublished trials. Additionally, hand-searching, Google Scholar searches, and contact with experts in the area were undertaken to identify potentially relevant published and unpublished studies. Inclusion criteria were (1) randomized controlled trials, case-control trials, or cohort studies with controls; (2) studies in nonsyndromic children 0 to 18 years of age with a diagnosis of sleep-disordered breathing or obstructive sleep apnea by either a sleep disorders unit, screening questionnaire, or polysomnography; and (3) principal outcome measures of craniofacial or upper airway dimensions or proportions with various modalities of imaging for the craniofacial and neck regions. The quality of the studies selected was evaluated by assessing their methodologies. Treatment effects were combined by meta-analysis with the random-effects method.
Results
Children with obstructive sleep apnea and primary snoring show increased weighted mean differences in the ANB angle of 1.64° ( P <0.0001) and 1.54° ( P <0.00001), respectively, compared with the controls. An increased ANB angle was primarily due to a decreased SNB angle in children with primary snoring by 1.4° ( P = 0.02). Children with obstructive sleep apnea had a distance from the posterior nasal spine to the nearest adenoid tissue measured along the PNS-basion line reduced by 4.17 mm (weighted mean difference) ( P <0.00001) and a distance from the posterior nasal spine to the nearest adenoid tissue measured along the line perpendicular to the sella-basion line reduced by 3.12 mm (weighted mean difference) ( P <0.0001) compared with the controls.
Conclusions
There is statistical support for an association between craniofacial disharmony and pediatric sleep-disordered breathing. However, an increased ANB angle of less than 2° in children with obstructive sleep apnea and primary snoring, compared with the controls, could be regarded as having marginal clinical significance. Therefore, evidence for a direct causal relationship between craniofacial structure and pediatric sleep-disordered breathing is unsupported by this meta-analysis. There is strong support for reduced upper airway width in children with obstructive sleep apnea. Larger well-controlled trials are required to address the relationship of craniofacial and upper airway morphology to pediatric sleep-disordered breathing in all 3 dimensions.
Sleep-disordered breathing is a disorder of breathing during sleep characterized by prolonged increased upper airway resistance, partial upper airway obstruction, or complete obstruction that disrupts pulmonary ventilation, oxygenation, or sleep quality. Pediatric sleep-disordered breathing is a continuum, with primary snoring at 1 end and complete upper airway obstruction, hypoxemia, and obstructive hypoventilation at the other, giving rise to obstructive sleep apnea.
Sleep-disordered breathing is associated with a wide variety of symptoms in children. Snoring is the most common nighttime symptom of sleep-disordered breathing in children. Chronic snoring, although common in adults, is considered abnormal in a pediatric population. Other symptoms associated with sleep-disordered breathing can include restless sleep, frequent arousals, snorting, gasping, unusual sleeping positions (eg, sitting), sweating during sleep, and nocturnal enuresis. The most prominent daytime symptom of sleep-disordered breathing in adults is excessive daytime sleepiness, which is absent in most children with polysomnography-proven obstructive sleep apnea. Sleep–disordered breathing in children is also associated with behavioral and impaired cognitive or school performance.
The current view is that adenotonsillar hypertrophy is the major cause of sleep-disordered breathing in otherwise normal healthy children. Adenotonsillar hypertrophy results in upper airway narrowing and, when superimposed with other factors (eg, reduced muscle tone), can lead to a clinically significant dynamic airway obstruction during sleep. Adenotonsillectomy is therefore often the first line of treatment for pediatric sleep-disordered breathing and is deemed curative in approximately 25% to 75% of patients. Nasal continuous positive airway pressure is often the next course of treatment, but there is emerging evidence of midface hypoplasia and other craniofacial side effects in children with this approach. There is currently no consensus on the best method of managing obstructive sleep apnea in childhood. Kaditis et al proposed a stepwise approach to treatment that starts with weight control and is followed by nasal corticosteroids, adenotonsillectomy surgery, orthodontic devices, continuous positive airway pressure, and, finally, craniofacial surgery or tracheostomy in severe cases.
Craniofacial disharmony can also be an important predisposing factor in the development and progression of pediatric sleep-disordered breathing. Studies in nonsyndromic children have shown a positive association between craniofacial disharmony and pediatric sleep-disordered breathing. Other contradictory studies, however, do not report such associations. There is no systematic review in the literature of the association between craniofacial and upper airway morphology in pediatric sleep-disordered breathing.
This aim of this study was to conduct a systematic review of the published and unpublished literature. A further aim was that the results of the primary studies would be combined by meta-analysis to statistically elucidate the nature of the association between craniofacial disharmony and pediatric sleep-disordered breathing. This will aid clinicians by increasing the diagnostic sensitivity of sleep-disordered breathing and might provide suggestions for alternative treatments for the children suffering from it.
Material and methods
Citations to potentially relevant trials published in journals and dissertations were located by searching the appropriate databases (PubMed, Embase, Scopus, and Cochrane Central Register of Controlled Trials). An effort to identify potentially relevant unpublished or ongoing trials was made by searching the MetaRegister of controlled trials database. Additionally, hand-searching, Google Scholar searches, and contact with experts in the area were undertaken to identify potentially relevant published and unpublished studies. The references cited in the reviewed articles were also checked. The search date was December 27, 2011, across all databases, and the search was updated monthly for PubMed and Scopus until April 2012. The Appendix Table shows the search strategy for this systematic review with a list of keywords used.
Inclusion criteria were limited to (1) randomized controlled trials, case-control trials or cohort studies; (2) studies of nonsyndromic children 0 to 18 years of age with a diagnosis of sleep-disordered breathing or obstructive sleep apnea by a sleep disorders unit, screening questionnaire, or polysomnography; and (3) principal outcome measures of craniofacial or upper airway dimensions or proportions with various modalities of imaging for the craniofacial and neck regions. The study selection criteria are given in Table I .
| Criterion | Definition |
|---|---|
| Study characteristics | The studies should be prospective or retrospective in design. Included study designs will be randomized controlled trials, case-control trials, or cohort studies with controls. |
| Patient characteristics | Nonsyndromic children, 0-18 years of age with a diagnosis of sleep-disordered breathing, primary snoring, or obstructive sleep apnea by a sleep disorders unit, screening questionnaire, or polysomnography. Studies in medically compromised patients and those studying craniofacial syndromes will be excluded. |
| Study method characteristics | Studies with various modalities of imaging for the craniofacial and neck regions in children will be included. |
| Outcome characteristics | Trials reporting outcome measures:
|
The primary author (V.K.) independently reviewed the titles and abstracts of all identified citations. Any studies not fulfilling the inclusion criteria were excluded from further evaluation, and the full articles were retrieved for those meeting the criteria. The primary author and a coauthor (C.N.D.) independently reviewed all full texts.
Data abstraction was performed independently by the same 2 authors using Excel (Microsoft, Redmond, Wash); this included year of publication, demographic details of the patients, details of the study design, the participants’ characteristics, the method of sleep-disordered breathing diagnosis, the measurement tool, a quality assessment, and the statistical details. Any disagreements were resolved by discussion and mutual agreement between the 2 authors. Angular variables were recorded in degrees (± standard deviations), and linear variables were recorded in millimeters (± standard deviations).
Statistical analysis
Revman (version 5.1; Nordic Cochrane Centre, Cochrane Collaboration; 2011) was used for the statistical analysis. The data categories common among the studies were used for the pooled analysis. Because of the expected variability in the trials, a random-effects model was chosen. To identify heterogeneity, the overlap of the 95% confidence intervals for the results of each study was inspected graphically, and the Cochrane test for homogeneity and the I 2 test were calculated to check for heterogeneity and inconsistency, respectively.
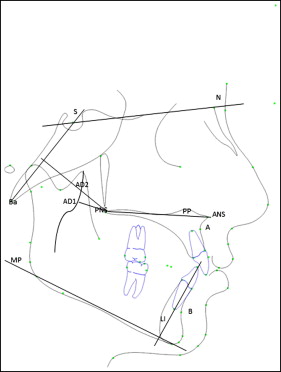
Forest plots to calculate the weighted mean differences were generated for the following cephalometric variables ( Fig 1 ) in children with obstructive sleep apnea: (1) SNA angle (angle between sella, nasion, and A-point), (2) SNB angle (angle between sella, nasion, and B-point), (3) ANB angle (difference of SNA and SNB angles), (4) SN-MP angle (angle made by the sella-nasion plane to the mandibular plane), (5) PP-MP angle (angle made by the palatal plane extending from ANS-PNS to the mandibular plane), (6) IMPA (angulation of the mandibular incisor to the mandibular plane), (7) BaSN angle (angle formed between basion, nasion, and sella), (8) PNS-AD1 (distance from the posterior nasal spine to the nearest adenoid tissue measured along the PNS-basion line), and (9) PNS-AD2 (distance from the posterior nasal spine to the nearest adenoid tissue measured along the line perpendicular to the sella-basion line). For children with primary snoring, the analysis was pooled for the SNA, SNB, ANB, and BaSN angles because of the limited data from the primary studies.
The planned subgroup analyses were based on age, sex, body mass index, and apnea-hypopnea index.
The quality of the studies selected was evaluated by assessing their methodologies. The assessment criteria were those from the Centre for Reviews and Disseminations in York, United Kingdom. These are presented in Table II .
| Strong evidence | Moderately strong evidence | Limited evidence |
|---|---|---|
|
|
|
Results
No restrictions were placed on year of publication. Restrictions were placed on the participants’ ages and the language. In the initial search, we found 875 citations across the 4 databases. Fourteen citations in foreign languages were excluded from this review. The search process is shown in Figure 2 . The characteristics of the 9 included trials, including their methodologic quality, are summarized in Table III . Only 2 trials reported blinding of observers to the diagnosis of children during data collection.
| Study | Banabilh et al (2008) | Cozza et al (2004) | Deng and Gao (2012) | Lofstrand-Tidestrom et al (1999) | Pirila-Parkkinen et al (2009) | Pirila-Parkkinen et al (2010) | Schiffman et al (2004) | Zettergren-Wijk et al (2006) | Zucconi et al (1999) |
|---|---|---|---|---|---|---|---|---|---|
| Design and participant characteristics | |||||||||
| Design | Prospective case control | Prospective case control | Prospective case control | Prospective cohort | Prospective case control | Prospective case control | Prospective case control | Prospective case control | Prospective case control |
| Total number of subjects | 60 (30 snorers and 30 controls) | 40 (20 OSA and 20 controls) | 30 (15 OSA and 15 controls) | 21 obstructed subjects and 40 controls for cephalometric examinations; 22 obstructed subjects and 48 controls for study model examinations | 123 (41 OSA, 41 snorers, and 41 controls) | 140 (70 subjects and 70 controls); subjects, 26 OSA, 27 snorers | 48 (24 OSA and 24 controls) | 34 (17 OSA and 17 controls) | 52 (26 OSA and 26 controls) |
| Mean age (y) ± SD or range (y); P, participants; C, controls; O, OSA; S, snorer | |||||||||
| P | 9.5 ± 2.47 | 5.91 ± 1.14 | 9.5 ± 1.0 | 4.52 ± 0.37 | (O) 7.2 ± 1.93 (S) 7.2 ± 1.79 | (O) 7.7 ± 1.91(S) 7.3 ± 1.61 | 4.9 ± 1.7 | 5.6 ± 1.34 | 4.6 ± 1.5 |
| C | 10.47 ± 2.28 | 6.00 ± 0.71 | 9.6 ± 1.8 | 4.58 ± 0.25 | 7.2 ± 1.90 | 7.3 ± 1.78 | 4.9 ± 1.8 | 5.8 ± 1.40 | 5.1 ± 0.5 |
| Sex distribution of subjects | |||||||||
| P | 16 M/14 F | 10 M/10 F | 11 M/4 F | – | (O) 22 M/19 F (S) 22 M/19 F | (O) 14 M/12 F (S) 9 M/18 F | 14 M/10 F | 10 M/7 F | – |
| C | 21 M/9 F | 10 M/10 F | 11 M/4 F | 20 M/20 F | 22 M/19 F | 34 M/36 F | 14 M/10 F | 10 M/7 F | – |
| Body mass index distribution of participants (kg/m 2 ) | |||||||||
| P | 21.22 ± 3.12 | 16.02 ± 3.40 | – | – | – | (O) 16.6 ± 3.46 (S) 16.8 ± 2.52 | Ht, 109 ± 13 Wt, 19.8 ± 5.7 | – | – |
| C | 21.42 ± 2.98 | 20.98 ± 0.48 | – | – | – | 16.6 ± 2.23 | Ht, 108 ± 13 Wt, 20.1 ± 5.5 | – | – |
| Controls matched for age and sex | No | Yes | Yes | Age matched | Yes | Yes | Yes | Yes | Age-matched |
| Methods used | |||||||||
| Method of sleep-disordered breathing diagnosis | Berlin questionnaire for subjects and controls | Overnight polysomnography and Epsworth sleepiness scale in subjects only | Polysomnography for subjects and controls | Polysomnography for subjects; historic controls (cephalometrics); controls from cohort study (study models) | Overnight polysomnography for OSA subjects and snorers only; controls selected by examinations parental reported histories | Overnight polysomnography for subjects only; controls selected by examinations and parental reported histories | Overnight polysomnography in subjects and 12 controls; Brouillette sleep questionnaire for control selection | Overnight polysomnography in subjects; 11 controls had ear-nose-throat examinations; 6 controls from growth study | Validated sleep questionnaire for all subjects and controls; diurnal polysomnography for subjects |
| Measurement tool: NHP, natural head position | Cephalogram | Cephalogram and dental models (width between centroids–Moyers method) | Cephalogram in NHP; magnification corrected | Cephalogram in NHP and dental models | Dental models (width between mesiolingual cusps—Moorrees method) | Cephalogram in NHP; magnification, 5% | Magnetic resonance imaging under intravenous sedation | Cephalogram | Cephalogram in NHP; magnification corrected |
| Error of the method: NS, not significant | NS | NS | 0.5°/0.5 mm | <1.1°/0.6 mm | NS | NS | NS | NS | NS |
| Study quality appraisal | |||||||||
| Evidence level (L, low; M, moderate) | L | M | M | M | M | M | M | M | L |
| Comments | Diagnosis by parental report; blinding not reported | Apnea, 10 s; OSA, apnea-hypopnea index, >1; blinding not reported | OSA: apnea-hypopnea index, >1; Bonferroni correction for statistics; blinding not reported | Subjects were not subdivided as snorers and OSA; results excluded from meta-analysis | Apnea, 10 s; OSA: apnea-hypopnea index, >1; blinding reported | Apnea, 10 s; OSA: apnea-hypopnea index, >1; blinding reported | Apnea, absence of oronasal thermistor signal for 2 respiratory cycles; blinding not reported | OSA: apnea-hypopnea index, >1; Bonferroni correction for statistics; blinding not reported | Apnea, 10 s; OSA: apnea-hypopnea index, >1; blinding not reported |
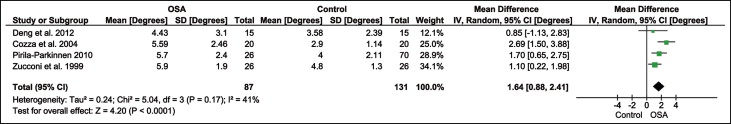
Children with obstructive sleep apnea and primary snoring showed increased weighted mean differences in the ANB angle of 1.64° ( P <0.0001) and 1.54° ( P <0.00001), respectively, in comparison with the controls ( Figs 3 and 4 ). Increased ANB was primarily due to decreased SNB angle in children with primary snoring by a weighted mean difference of 1.4° ( P = 0.02) ( Fig 5 ). Children with obstructive sleep apnea had a PNS-AD1 distance reduced by 4.17 mm (weighted mean difference) ( P <0.00001) and a PNS-AD2 distance reduced by 3.12 mm (weighted mean difference) ( P <0.0001) compared with the controls ( Figs 6 and 7 ).
The weighted mean differences in the SNA, SNB, SN-MP, PP-MP, IMPA, and BaSN for children with obstructive sleep apnea compared with the controls are given in Appendix Figures 1 through 6 , respectively. The weighted mean differences in the SNA and BaSN angles for children with primary snoring compared with the controls are shown in Appendix Figures 7 and 8 , respectively.
The pooled cephalometric variables in children with obstructive sleep apnea and primary snoring are summarized in Tables IV and V , respectively. There was significant heterogeneity for the variables SN-MP ( P = 0.04) and PP-MP ( P <0.00001) in children with obstructive sleep apnea. The increased weighted mean difference in the SN-MP angle of 2.74° ( P = 0.006) might indicate a trend toward increased lower anterior face height in pediatric obstructive sleep apnea patients. However, this result must be interpreted with caution because of its borderline heterogeneity.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


