Several sedation options are used to minimize pain, anxiety, and discomfort during oral surgery procedures. Minimizing or eliminating pain and anxiety for dental care is the primary goal for conscious sedation. Intravenous conscious sedation is a drug-induced depression of consciousness during which patients respond purposefully to verbal commands. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate as well as cardiovascular function. Patients must retain their protective airway reflexes, and respond to and understand verbal communication. The drugs and techniques used must therefore carry a broad margin of safety.
Key points
- •
The use of intravenous (IV) conscious sedation in dentistry has gained significant popularity over the last decades to help manage pain and anxiety in the dental office setting.
- •
The goals of successful sedation should include a physical and psychological evaluation. The plan should be realistic and should (1) determine the patient’s physical status and length of the procedure, (2) determine the patient’s psychological status, (3) determine whether sedation is indicated, (4) determine whether treatment modifications are needed, (5) determine which drug regimen is appropriate, and (6) determine whether contraindications exist for conscious sedation or the drugs to be used.
- •
IV conscious sedation is also referred to as parenteral or moderate sedation.
- •
Moderate sedation is defined as a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate as well as cardiovascular function.
- •
For practitioners providing moderate sedation in their offices, it is imperative that they are knowledgeable about guidelines and are adequately trained to safely administer moderate sedation. Further, practitioners and their staff providing sedation need requisite training in basic life support, advanced cardiac resuscitation, and/or pediatric advanced cardiac resuscitation techniques.
Introduction
Thanks to the efforts of Dr Horace Wells and his student, Dr William T.G. Morton, sedation has become an integral part of the practice of dentistry. Dr Wells is credited with the introduction of nitrous oxide, or laughing gas, as a way to control pain and anxiety during dental procedures. To demonstrate the effectiveness of nitrous oxide during dental surgery, on December 11, 1844, Dr Wells successfully used nitrous oxide on himself while having a colleague extract one of his wisdom teeth. The following year he performed a similar experiment at Harvard using a real patient and the patient cried out during the procedure, resulting in Wells being ridiculed by those attending the lecture. Although Wells was not as successful as he had hoped, a former student 2 years later, Dr William T.G. Morton, successfully administered sulfuric ether to a patient in front of a group of physicians and students at Harvard University to remove a tumor. The combined efforts of these two pioneers introduced the spectrum of sedation that is, used to effectively minimize pain, anxiety, and discomfort during oral surgery procedures. Minimizing or eliminating pain and anxiety for dental care is the primary goal for conscious sedation.
This article provides dental practitioners, surgeons, and recent graduates with a review of the literature and the most up-to-date guidelines and methods used in the practice of intravenous (IV) conscious sedation. Goals of successful sedation should include a physical and psychological evaluation. The plan should be realistic and should:
- 1.
Determine the patient’s physical status and the length of the procedure
- 2.
Determine the patient’s psychological status
- 3.
Determine whether sedation is indicated
- 4.
Determine whether treatment modifications are needed
- 5.
Determine which drug regimen is appropriate
- 6.
Determine whether contraindications exist for conscious sedation or the drugs to be used
New guidelines for training and monitoring have emerged. The American Society of Anesthesiologists (ASA) currently mandates that “during moderate or deep sedation, the adequacy of ventilation shall be evaluated by continual observation of qualitative clinical signs and monitoring for the presence of exhaled carbon dioxide unless precluded or invalidated by the nature of the patient, procedure, or equipment.” This guideline is one of the most recent that is discussed in this article.
Introduction
Thanks to the efforts of Dr Horace Wells and his student, Dr William T.G. Morton, sedation has become an integral part of the practice of dentistry. Dr Wells is credited with the introduction of nitrous oxide, or laughing gas, as a way to control pain and anxiety during dental procedures. To demonstrate the effectiveness of nitrous oxide during dental surgery, on December 11, 1844, Dr Wells successfully used nitrous oxide on himself while having a colleague extract one of his wisdom teeth. The following year he performed a similar experiment at Harvard using a real patient and the patient cried out during the procedure, resulting in Wells being ridiculed by those attending the lecture. Although Wells was not as successful as he had hoped, a former student 2 years later, Dr William T.G. Morton, successfully administered sulfuric ether to a patient in front of a group of physicians and students at Harvard University to remove a tumor. The combined efforts of these two pioneers introduced the spectrum of sedation that is, used to effectively minimize pain, anxiety, and discomfort during oral surgery procedures. Minimizing or eliminating pain and anxiety for dental care is the primary goal for conscious sedation.
This article provides dental practitioners, surgeons, and recent graduates with a review of the literature and the most up-to-date guidelines and methods used in the practice of intravenous (IV) conscious sedation. Goals of successful sedation should include a physical and psychological evaluation. The plan should be realistic and should:
- 1.
Determine the patient’s physical status and the length of the procedure
- 2.
Determine the patient’s psychological status
- 3.
Determine whether sedation is indicated
- 4.
Determine whether treatment modifications are needed
- 5.
Determine which drug regimen is appropriate
- 6.
Determine whether contraindications exist for conscious sedation or the drugs to be used
New guidelines for training and monitoring have emerged. The American Society of Anesthesiologists (ASA) currently mandates that “during moderate or deep sedation, the adequacy of ventilation shall be evaluated by continual observation of qualitative clinical signs and monitoring for the presence of exhaled carbon dioxide unless precluded or invalidated by the nature of the patient, procedure, or equipment.” This guideline is one of the most recent that is discussed in this article.
Moderate/conscious sedation
IV conscious sedation, also referred to as parenteral or moderate sedation, is defined as a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate, as well as cardiovascular function. In addition, patients must retain their protective airway reflexes, and be able to respond to and understand verbal communication. The drugs and techniques used must therefore carry a margin of safety broad enough to make loss of consciousness and airway control unlikely.
Conscious sedation is intended to allow the patient to maintain protective reflexes, but sedation represents a continuum and at times an individual patient may experience a deeper sedation than was anticipated. It is extremely important that the practitioner has the requisite knowledge, training, and skill to manage all levels of sedation adequately, identify unintended outcomes, and manage an emergency until either assistance arrives or the patient is successfully recovered to baseline status. Therefore, understanding the levels of anesthesia is helpful in guiding provider in selection of the proper sedation technique and drugs. The levels of anesthesia are listed in Table 1 .
| Minimal or anxiolysis | A drug-induced state during which patients respond normally to verbal commands. Although cognitive function and physical coordination may be impaired, airway reflexes, ventilation, and cardiovascular functions are unaffected |
| Moderate or conscious sedation | A drug-induced depression of consciousness during which patients respond purposefully** to verbal commands, either alone or accompanied by light tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate. Cardiovascular function is usually maintained |
| Deep/analgesia | A drug-induced depression of consciousness during which patients cannot be easily aroused but respond purposefully following repeated or painful stimulation. The ability to independently maintain ventilation function may be impaired. Patients may require assistance in maintaining a patent airway, and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained |
| General | A drug-induced loss of consciousness during which patients cannot be aroused, even by painful stimulation. The ability to independently maintain ventilatory function is often impaired. Patients often require assistance in maintaining a patent airway, and positive pressure ventilation may be required because of depressed spontaneous ventilation or drug-induced depression of neuromuscular function. Cardiovascular function may be impaired |
The use of IV conscious sedation in dentistry has gained significant popularity over the last decades. Along with this popularity has come continued concerns with deaths associated with administration of conscious sedation as well as the need for adequate training/guidelines for practitioners and their staff to improve patient safety in the dental office setting. Although morbidity and mortality outcomes still exist, the extent of adverse outcomes is not clearly documented in the literature. A study published by the Journal of the American Dental Association in 2001 comparing 4 IV sedation drug regimens in 997 patients concluded that the drugs and doses evaluated were of therapeutic benefit in the outpatient setting and there was minimal incidence of potentially serious adverse effects. This study helped to reinforce the safety of the use of conscious sedation using different drug combinations with careful titration and adequate provider training. In contrast, a more recent study published in the Journal of Public Safety by Karamnov and colleagues, in a retrospective review conducted on 143,000 moderate sedation cases performed outside the operating room, showed that adverse events were associated with patient characteristics and procedure types. Patient harm was associated with age, body mass index (BMI), comorbidities, female sex, and gastroenterology procedures. Having a good working knowledge of pharmacodynamics, titration of medications to the adequate level of sedation, and strict guidelines, along with use of monitoring devices, has had a significant impact on patient safety and improved outcomes in conscious sedation (discussed later).
Even with improved practice guidelines and knowledge, adverse outcomes have not been eliminated. Guidelines established by the ASA in 2001 and updated in 2002 provided the foundation for provision of sedation in most practice settings. In addition to the ASA, the American Association of Oral and Maxillofacial Surgeons, the American Dental Association (ADA), and the American Academy of Pediatric Dentistry (AAPD) have all developed sedation guidelines relating to administering sedation during dental and surgical procedures as well as the requisite education and skills.
The ASA task force for the establishment of guidelines for monitoring patient sedation by nonanesthesiologists in 1996 replaced conscious sedation with the more precise term sedation-analgesia, but the term conscious sedation continues to be widely used, along with the term moderate sedation. The ADA has also produced several documents to guide the use of sedation for dental practitioners that include Guidelines for the Use of Sedation and General Anesthesia by Dentists, Guidelines for Teaching Pain Control and Sedation to Dentists and Dental Students, and ADA Policy Statement: The Use of Sedation and General Anesthesia by Dentists. Similar to the ASA, the ADA provides a definition for moderate sedation in the dental office setting.
Recently underway, the ADA is in the process of updating the guidelines for sedation. The ADA Council on Dental Education and Licensure has called for comments and input from its communities of interest regarding the anesthesia guidelines, with an imposed deadline of June 29, 2015. Some of the proposals recommend changes in definitions, educational requirements, terminology, and clinical and educational guidelines. For example, under section I, Definitions, the definition given earlier is recommended to be modified as follows “moderate sedation – a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, ….” The following definition applies to the administration of moderate or greater sedation: “titration – administration of incremental doses of a drug until a desired effect is reached. Knowledge of each drug’s time of onset, peak response and duration of action is essential to avoid over sedation. Although the concept of titration of a drug to effect is critical for patient safety, when the intent is moderate sedation one must know whether the previous dose has taken full effect before administering an additional drug increment.” In addition, a recommended change under section III, Education Requirements, states that to administer moderate sedation, the dentist must “demonstrate competency”; this reference to competency has been newly added. The guidelines for conscious sedation administration and training differ only slightly between most governing bodies. Credentialing is required by most dental boards nationally and some internationally and it is imperative that practitioners providing this service are knowledgeable about guidelines and adequately trained to safely administer moderate sedation. Further, practitioners and their staff providing sedation need requisite training in basic life support, advanced cardiac resuscitation (ACLS), and/or pediatric advanced cardiac resuscitation techniques.
Preoperative assessment
Having a satisfactory outcome from IV sedation and anesthesia greatly depends on the experience of the provider, patient selection, and the sedation plan (preoperative and postoperative). Having an approach to sedation that is consistent is valuable for obtaining predictable outcomes during and after the procedure. It is critical that the approach to sedation involves a preoperative evaluation of the patient that includes a comprehensive medical and dental history and physical examination. Additional information should include an anesthesia history and any record of adverse reactions to sedation or anesthesia. The patient should be queried on past medical history involving any major medical problems and systems disease and family history of disease. The patient should also provide a list of past surgeries, food and drug allergies, and a list of current medications. A report of current or past history of drug use or abuse should also be obtained, including history of smoking and alcohol use.
Medical History, Dental and Physical Examination
A complete and comprehensive medical and dental examination, including family history, should be taken and should cover all systems. Any positive responses should be thoroughly discussed and documented. The history is divided into major sections: the chief complaint, history of present illness, past medical history, review of systems, family history, and social history. Past medical history review elaborates on medical dental and psychological illnesses, hospitalizations, experiences with anesthesia, past surgeries, medication, and allergies. Review of systems involves gathering information covering general health, head, ears, eyes, nose, and throat, and signs and symptoms of diseases involving the cardiovascular, respiratory, gastrointestinal, genitourinary, integumentary, nervous, psychiatric, endocrine, hematologic, and musculoskeletal systems, and medical treatments (drugs and other physiologically active compounds). Patients with conditions of most concern should be evaluated more closely when considering IV sedation. The aging of the population and advancements in technology and medical discovery have resulted in an increase in the number of individuals living with chronic diseases. Significant among them are about 610,000 Americans who die from heart disease each year, which represents 1 in every 4 deaths and, depending on the extent of disease, presents significant challenges for delivery of conscious sedation. Conditions of most concern in this group are ischemic disease resulting in angina, myocardial infarction, heart failure, valvular disease, and cardiac arrhythmias. Liver disease, renal function, and respiratory disorders are also important to evaluate as they relate to administering conscious sedation because of concerns of drug administration/overdose and metabolism, as well as airway compromise. In addition to the medical history, any adverse reaction to anesthesia should be discussed with the patient. Most individuals who have had problems with anesthesia are able to recall most details concerning the incident. For individuals who have not been exposed to sedation or anesthesia, family history may be helpful in identifying those at risk for complications with sedation. Information concerning past hospitalizations and surgeries is also helpful in determining the presence of disease and the degree of severity before developing a sedation plan.
Medications
The medications list is also an essential component of the medical history. It provides valuable insight into the patient’s medical status and possible drug interactions. The inquiry should include medications that are prescribed as well as those that are over-the-counter, alternative, or homeopathic medications. The need to discontinue medications before IV sedation is generally not indicated. However, there are certain medications that may require the practitioner to alter the sedation plan by supplementation or altering drug dosages. Chronic glucocorticoid use, insulin use, anticoagulant therapy, and the use of sympathomimetics may increase risks if not managed properly before the sedation appointment. Allergies to any medications or foods should be evaluated as well. Reported allergies should be investigated and the clinician should determine whether the reaction is related to delayed hypersensitivity or is an immunoglobulin E–mediated response.
If the allergy cannot be clearly delineated, the patient may need to be referred for allergy testing.
Physical Evaluation
The physical examination requires collection of baseline information such as vital signs (blood pressure, pulse and oxygen saturation). Also of importance are patient characteristics such as age, weight, height, and BMI. Once a complete history is obtained for the patient’s physical status, the patient is assigned a classification based on the ASA classification system developed in 1941 and revised in 1984. This scale has been used widely and a recent study concluded that the scale has inherent subjectivity, with moderate inter-rater reliability in clinical practice, and also shows validity as a marker of a patient’s preoperative health status.
The classification system is still widely used and has been shown to be effective in evaluating physical status for sedation and general anesthesia. The classification system is shown in Table 2 .
| ASA I | Healthy (ie, nonsmoking, no or little alcohol use) |
| ASA II | Mild systemic disease (ie, current smoker, well-controlled disease, pregnancy, obesity) |
| ASA III | Severe systemic disease (ie, 1 or more moderate to severe diseases) |
| ASA IV | Severe systemic disease that is a constant threat to life (ie, unstable disease myocardial infarction, cardiovascular accident) |
| ASA V | Patient who is not expected to survive without surgical intervention |
| ASA VI | Organ donor, patient is brain dead *The addition of E denotes emergency surgery (an emergency is defined as existing when delay in treatment of the patient would lead to a significant increase in the threat to life or body part) |
Patients who are classified as ASA I or II may receive a physical that is focused on sedation, whereas those at III and IV or with more unstable disease may require a more comprehensive evaluation. Based on ASA recommendations, review of the medical history and medications should take place for healthy or medically stable individuals (ASA I or II) within 30 days. Also, individuals with significant medical considerations (ASA III or IV) may require consultation with their primary care physicians or consulting medical specialists, including an immediate preoperative review before administration of sedation. Along with the physical examination and classification of physical status, the airway needs to be examined and scored to ensure that a patent airway is available before the sedation appointment. This step is essential to the preoperative evaluation. The Mallampati or Modified Mallampati airway system of classification is most commonly used and is the standard of care ( Fig. 1 , Table 3 ). This indirect approach originally consisted of 3 categories, and a fourth category was added by Samsoon and Young in 1987 creating the modified scale. An additional modification has been proposed that would expand the scale with class 0, which is defined as the ability to see any part of the epiglottis on mouth opening and tongue protrusion. Other systems used to classify airway difficulty include the Cormack-Lehane classification system, the Simplified Airway Risk Index, and thyromental distance.
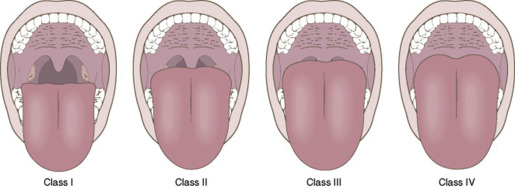
| Class I | Complete visualization of the uvula, tonsillar pillars, and soft palate |
| Class II | Partial visibility of the uvula and complete soft palate |
| Class III | Only the soft palate is visible |
| Class IV | Only the hard palate is visible |
Increases in score generally result in increases in risks in management of the airway. Any airway disorders should be documented, as well as severity. Individuals with obstructive sleep apnea or reactive airway disorders such as severe asthma or chronic obstructive pulmonary that are not stable may not be candidates for moderate sedation in the office setting.
Airway class III or VI classifications can be more difficult to manage under deeper levels, are at greater risk for obstruction, and are more difficult to intubate. Preoperative instructions should cover preoperative fasting to avoid airway compromise and possible aspiration. The guidelines provided by the ASA should be closely adhered to in providing conscious sedation to avoid potential complications ( Table 4 ).
| Clear liquids | 2 h |
| Breast milk | 4 h |
| Infant formula | 6 h |
| Nonhuman milk | 6 h |
| Light meal | 6 h |
| Fatty | 8 h |
Pharmacotherapeutics
All doses of medication given should be verified with the manufacturer’s package insert. In addition, all drugs to be administered should be properly labeled before the sedation procedure. Doses outlined the article for medications should not be relied on as accurate or definitive. Dosing should be based on the individual patient.
Inhalation Agents
Nitrous oxide
Nitrous oxide is an inhaled anesthetic. Its potency is defined by the minimum alveolar concentration (MAC) that produces immobility to a skin incision in 50% of the patients who are subjected to such stimuli. The MAC for nitrous oxide at 1 atm in adults is 104%. It has a blood gas partition coefficient of 0.47. When nitrous oxide and a potent inhalation anesthetic are given concurrently, the wash-in of the anesthetic administered in a small concentration may be increased if the uptake of the second anesthetic is large. However, more recent evidence suggests that this second gas effect may not have any clinical significance and, if it does exist, it is minimal. Another well-known phenomenon associated with nitrous oxide is the concept of diffusion hypoxia. When the surgical procedure is completed and the inhalation gases are turned off this may be seen during the first 10 minutes of recovery. There is a rapid outflow of nitrous oxide, and this was originally called diffusion anoxia by Fink. The 2 mechanisms thought to be responsible for the hypoxia are a direct displacement of oxygen and a diluting of carbon dioxide in the alveolar compartment by the outflow of nitrous oxide, thereby decreasing the respiratory drive and ventilation.
Many anesthesiologists administer 100% O 2 during the first 5 to 10 minutes of recovery. Nitrous oxide is contraindicated in patients with pneumothorax or in procedures in which air embolus is a risk, as well as in middle ear surgical procedures. Nitrous oxide is 34 times more soluble than nitrogen in blood. As mentioned earlier, it has a blood gas partition coefficient of 0.47 at 37°C. It defuses into cavities that contain nitrogen more rapidly than nitrogen escapes, thereby increasing the volume of the cavity. Nitrous oxide can enter any gas-filled cavity, such as obstructed bowel, pneumothorax and endotracheal tube cuffs, and bubbles in veins, and it should be avoided in laparoscopic surgery. The National Institute of Occupational Safety and Health set a limit of chronic exposure to nitrous oxide as 25 ppm because of its effects on organ systems and teratogenicity. Nitrous oxide has a rapid onset of less than 5 minutes and when it is discontinued the patient’s return to baseline status is rapid. When nitrous oxide is combined with midazolam or fentanyl, alone or in combination, a deeper level of sedation can be reached with lower dosages of the benzodiazepine or narcotic required. Fifty percent nitrous with oxygen can produce minimal sedation and 70% nitrous combined with oxygen can produce moderate sedation ( Fig. 2 ).
Sedative-Hypnotic Agents
Sedative-hypnotics are drugs that depress or slow down the body’s functions. Their effects range from calming down anxious people to promoting sleep. At high doses, the drugs can cause unconsciousness and death. Barbiturates and benzodiazepines are the two major categories of sedative-hypnotics.
Benzodiazepines
Diazepam (Valium) and midazolam (Versed) are widely used in dentistry for moderate sedation. Midazolam is the first synthesized water-soluble benzodiazepine. They both are lipid soluble at physiologic pH, with midazolam being more lipid soluble in vivo. Each milliliter of diazepam (5 mg) contains 0.4 mL of propylene glycol, 0.1 mL of alcohol, 0.015 mL of benzyl alcohol, and sodium benzoate/benzoic acid in water for injection (pH 6.2–6.9). Midazolam is formulated with 1 mg or 5 mg/mL of midazolam plus 0.89% sodium chloride and 0.019% disodium edetate, with 1% benzyl alcohol as a preservative. The pH is adjusted to 3 with hydrochloric acid and sodium hydroxide. As noted earlier, midazolam’s lipid solubility is pH dependent and, because of its pH- dependent solubility, it is water soluble when formulated in its buffered acidic medium at pH 3.5. Because it is highly lipophilic, it has a fast onset in the central nervous system (CNS) and a large volume of distribution. The benzodiazepines are metabolized in the liver. Midazolam is considered a short-acting benzodiazepine and diazepam a longer lasting benzodiazepine based on their metabolism and clearance. The patient’s age and weight, and function of the patient’s hepatic and renal systems all affect the duration of action and effect of the drug.
The benzodiazepines all have amnesic, hypnotic, sedative, anxiolytic, anticonvulsant, as well as centrally produced muscle relaxant properties. Midazolam is 3 to 6 times as potent as diazepam. The benzodiazepines occupy the gamma-aminobutyric acid (GABA) receptor. GABA is the major inhibitory neurotransmitter in the brain. By occupying the GABA receptor the benzodiazepines exert their effect and the percentage of receptors occupied determines the effect that is seen. A benzodiazepine receptor with less than 20% occupancy may have the ability to produce a decrease in anxiety; 30% to 50% of occupied receptor sites may show sedation and greater than 60% of occupied sites produce unconsciousness. Midazolam binds to the GABA A receptor and then there is a chloride ion influx and hyperpolarization and the cell becomes resistant to neuronal excitation. Benzodiazepines decrease cerebral blood flow and increase the seizure threshold of local anesthetics in mice exposed to lethal doses of anesthetics.
Benzodiazepines decrease respiratory rate and, when combined with opioids, there is a greater effect on respiratory depression. Midazolam can cause a minimal lowering of arterial blood pressure. The combination of nitrous oxide and midazolam has minimal hemodynamic effects compared with the combination of opioids and benzodiazepines, which can have a significant effect by lowering blood pressure. Patients presenting to the dental office may be nervous or anxious about the visit or procedure, especially if it involves a surgical procedure or any procedure in which injections are required. Aside from inhalation with nitrous oxide, midazolam could be considered preoperatively in the oral form for pediatric patients or intravenously depending on the patient’s willingness to accept an IV line to decrease anxiety and produce amnesia. By implementing nitrous oxide into the sedation technique, clinicians can achieve a cutaneous feeling of numbness of the extremities, which provides a more pleasant experience when placing the catheter for the IV line. A new IV fluid bag appropriate for the patient (for injection only) is chosen and always attached to a sterile disposable tubing line that runs from the fluid bag to the already placed disposable angiocatheter. All tubing, catheters, and syringes are disposed of after the procedure. (Note: All doses of medication should be verified with the manufactured package insert. The doses noted in this section for medication should NOT be relied upon as accurate nor definitive). Midazolam intravenously has a rapid onset because of its lipid solubility, and its peak effect is in about 2 to 4 minutes. The adult dose is 0.5 mg to 1.0 mg IV administered over 2 minutes and titrated until the desired level of sedation is obtained. The pediatric dose range is 0.025 mg/kg IV to 0.5 mg/kg IV with an onset of about 1 to 3 minutes and duration of action of 45 to 60 minutes intravenously. Using midazolam in children may produce hyperexcitability and further anxiousness to the point of combativeness. The level of consciousness may not correlate with the amnesia effect of the benzodiazepines. Patients may be awake or seem alert during the procedure but have no recall of it when questioned postoperatively. Midazolam is contraindicated in patients with acute narrow-angle glaucoma or a hypersensitivity to the drug. Benzodiazepines can cause respiratory depression and upper airway obstruction. In children, respiratory depression may be significant, especially in patients with enlarged tonsils. The combination of opioid and benzodiazepines in children has, as expected, an additive effect so the total effect is greater than the effect of each individual drug. Midazolam produces anterograde amnesia. Children who had dental extractions with midazolam better tolerated additional dental treatment than those treated without midazolam.
Barbiturates
Barbiturates have the basic structure of barbituric acid. These drugs act as CNS depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. Barbiturates are a family of compounds that have sedative and hypnotic activities and act as nonselective CNS depressants. The GABA receptor is one of barbiturates’ main sites of action, and therefore it is thought to play a pivotal role in the development of tolerance to and dependence on barbiturates. The most common use for barbiturates currently is as anesthesia for surgery. Current indications for the barbiturates include short-term treatment of insomnia and as a preanesthetic agent. Amobarbital (Amytal) and butabarbital (Soneryl, Butisol) are currently available short-acting barbiturates for parenteral administration, being used largely as preanesthetic agents. Other short-acting agents in use include secobarbital, which is available as a 100-mg capsule generically and under the brand name Seconal; it is also classified as a schedule II substance because there is the potential for physical and psychological dependence and abuse. Very-short-acting drugs in use include pentobarbital (Nembutal), methohexital (Brevital), and thiopental (Pentothal). Further medium-acting agents, such as butalbital (Fiorinal, Fioricet), Talbutal (Lotusate) and long-acting mephobarbital (Mebaral) and methylphenobarbital (Prominal), are available.
Barbiturates induce several hepatic cytochrome P (CYP) enzymes (most notably CYP2C9, CYP2C19, and CYP3A4), leading to exaggerated effects from many prodrugs and decreased effects from drugs that are metabolized by these enzymes to inactive metabolites. This property can result in fatal overdoses from drugs such as codeine, tramadol, and carisoprodol, which become considerably more potent after being metabolized by CYP enzymes. Although all known members of the class possess relevant enzyme induction capabilities, the degree of inhibition overall, as well as the impact on each specific enzyme, span a broad range, with secobarbital being the most potent enzyme inducer and butalbital and talbutal being among the weakest enzyme inducers in the class.
In addition to their sedative-hypnotic properties, this class also has anxiolytic and anticonvulsant properties. Barbiturates also have analgesic effects; however, these effects are weak, preventing barbiturates from being used in surgery in the absence of other analgesics. They have addiction potential, both physical and psychological. Barbiturates have now largely been replaced by benzodiazepines in routine medical use, mainly because benzodiazepines are significantly less dangerous in overdose because there is no specific reversal agent for barbiturate overdose. The longest-acting barbiturates have half-lives of a day or more, and subsequently result in bioaccumulation of the drug in the system. The therapeutic use of long-acting barbiturates wears off significantly faster than the drug can be eliminated, allowing the drug to reach toxic concentrations in the blood following repeated administration (even when taken at the therapeutic/prescribed dose) despite the user feeling little or no effect from the plasma-bound concentrations of the drug. Individuals who consume alcohol or who are given sedatives after the drug effects have worn off, but before it has cleared the system, could experience an exaggerated effect from the sedatives, which can be incapacitating or even fatal.
There are special risks to consider for older adults, women who are pregnant, and babies. When a person ages, the body becomes less able to rid itself of barbiturates. As a result, people more than 65 years of age are at higher risk of experiencing the harmful effects of barbiturates, including drug dependence and accidental overdose. A rare adverse reaction to barbiturates is Stevens-Johnson syndrome, which primarily affects the mucous membranes.
Barbiturates in overdose with other CNS depressants (eg, alcohol, opiates, benzodiazepines) are even more dangerous because of additive CNS and respiratory depressant effects. In the case of benzodiazepines, not only do they have additive effects but barbiturates also increase the affinity of the benzodiazepine binding site, leading to exaggerated benzodiazepine effects. Frequent side effects include drowsiness, sedation, hypotension, nausea, headache, and skin rash.
Propofol (Diprivan)
Although this article is about moderate sedation, propofol should also be mentioned. Propofol use often causes patients to be in a state of deep sedation or general anesthesia, with the inability of the patient to maintain an airway continuously and independently. Therefore propofol may be considered an agent that often produces a level of deep sedation/general anesthesia and therefore should not be used in a facility where only moderate sedation is approved. Regardless, all facilities providing sedation, whether moderate or deep, should be prepared to manage the complications that may arise, including, but not limited to, airway compromise and apnea. Propofol is a sedative-hypnotic that is used for the induction and maintenance of anesthesia. Diprivan is composed of 1% propofol, 10% soybean oil, 1.25% egg yolk phosphatide, 2.25% glycerol, ethylenediaminetetraacetic acid, and sodium hydroxide to maintain a pH of 7.0 to 8.5. It is highly lipophilic with a rapid distribution to vessel-enhanced organs and therefore has a rapid induction. It has rapid redistribution and hepatic and extrahepatic clearance, which is why it has a short duration of action and requires frequent repeated doses or a continuous infusion to maintain the desired level of anesthesia. Propofol is a sedative-hypnotic and its effects on the CNS is thought to be the result of increasing the GABA-induced chloride current through binding to the beta subunit of the GABA A receptor.
Propofol inhibits acetylcholine release by its action on GABA A receptors in the hippocampus and prefrontal cortex. This acetylcholine release inhibition is thought to be responsible for the sedative effect of propofol. Propofol also has an inhibitory effect on the N -methyl- d -aspartate (NMDA) receptor via the sodium channel, which may also contribute to the action of the drug on the CNS. Propofol has antiemetic properties and produces a sense of well-being. Propofol decreases intraocular pressure, as well as intracranial pressure. Propofol can cause a decrease in respiratory rate and apnea. Propofol causes bronchodilation in patients with chronic obstructive pulmonary disease. There is a decrease in arterial blood pressure seen with propofol. It has both a depressant and vasodilation effect on the heart that may be dose and plasma concentration related.
Propofol used for sedation is best administered by an infusion pump but incremental dosing also can be done. An infusion rate for sedation in which local anesthesia is used in healthy adults is 30 to 60 μg/kg/min. In pediatric patients the dosage required can range from a bolus of 1 to 2 mg/kg with an infusion rate of 50 to 250 μg/kg/min. With propofol there may be pain on injection, hypotension, and apnea on induction. The use of an opioid along with propofol increases the incidence of apnea, as well as decreasing the arterial blood pressure. In pediatric patients the arterial blood pressure was decreased more, and the total dosage was greater, when an infusion was used compared with intermittent boluses. In pediatric patients, IV lidocaine should be considered to relieve the pain associated with injection. Bradycardia can be seen in both adults and children with propofol. Propofol has a negative effect on airway patency and respiration in children. The airway narrows in children during infusion but remains patent. All open vials of propofol must be discarded within 6 hours because of the potential growth of Escherichia coli , Staphylococcus aureus , Pseudomonas aeruginosa , and Candida albicans . Egg allergy in adults and children is not considered a contraindication to propofol use; however, it is recommended to avoid propofol in children with documented anaphylaxis to eggs.
Narcotics Analgesics
Opioids
Morphine is the prototype opioid for all other opioids. Previously, it was an integral part of the sedation regimen for prolonged procedures, but morphine has no application in modern IV sedation procedures. Its main usefulness is in acute pain management. The onset of morphine is slow: 5 to 10 minutes following IV administration and up to 20 minutes following intramuscular injection. It produces analgesia, euphoria, and sedation lasting from 2 to 4 hours. Its use is limited by side effects such as histamine release, postural hypotension, and nausea and vomiting.
Meperidine (Demerol) is the prototype of the phenylpiperidine series of opioids, which includes fentanyl, sufentanil, alfentanil, and remifentanil. Meperidine is an opioid narcotic that binds to opioid receptors in the CNS. The dose for adults is generally 25 to 50 mg in incremental doses to a maximum dose of 150 mg; for elderly patients (65 years and older) the dose is 25 mg in incremental doses to a maximum dose of 75 mg. The elderly are more susceptible to CNS depression. In addition, they are more susceptible to seizures from accumulation of normeperidine, a metabolite of meperidine, as a result of reduced renal function. For years meperidine was the mainstay of IV sedation regimens for procedures of all durations. It has a more rapid onset than morphine, within 3 to 5 minutes following IV administration, making it easier to titrate than morphine. The peak effect is 1 hour, and duration of action is 2 to 4 hours. Meperidine is 10 times less potent than morphine, producing sedation and analgesia lasting 45 to 90 minutes. Meperidine was first investigated as an atropinelike agent and is unique among opioids in that it may produce tachycardia and drying of secretions. It also releases histamine, and may produce orthostatic hypotension with rapid position change. Severe asthma is a relative contraindication. Other side effects include dysphoria, especially in the absence of pain, and nausea and vomiting. Meperidine is associated with increased neuronal activity that may result in CNS excitation. Its metabolite, normeperidine, is twice as potent as meperidine in producing CNS excitation and convulsions. Meperidine is contraindicated in patients taking monoamine oxidase inhibitors because concentrations of normeperidine are increased with these drugs. Although meperidine is still used on a limited basis for dental sedation, its main use is currently in the management of postanesthetic shivering. Opioids in general reduce thermoregulation thresholds similarly to potent inhalational agents.
Fentanyl, sufentanil, alfentanil, and remifentanil are synthesized opioid compounds that are phenylpiperidine derivatives. The brain and spinal cord contains the mu receptors, which are responsible for modulating the effects of opioids. Opioids produce analgesia, decrease respiratory drive, and increase sedation at the mu receptor. Neuronal excitation is decreased by the action of the opioids on the receptors, which depends on the suppression of the Ca 2+ channel and activation of the K + channel.
Opioids decrease cerebral blood flow with nitrous oxide. Giovannitti in 2013 provided a table comparing common drugs in this class in terms of potency, peak effect, duration, and half-life ( Table 5 ). There are reports of no significant effect on intracranial pressure in patients with head trauma and opioids have been used safely in such patients. Other reports have shown a slight increase in intracranial pressure in head injured patients with morphine and fentanyl. The increase in intracranial pressure is thought to be multifactorial. Muscle tone and muscle rigidity can be increased with opioids. The rigidity can lead to severe respiratory problems. In an awake patient this may be shown by hoarseness. It also can be shown just as, or immediately after, a patient losses consciousness. The muscle rigidity is not caused by a direct action on the muscle fiber, but is thought to be CNS regulated; especially the nucleus pontes raphae. The closure of vocal cords is thought to be the reason for difficulty in the ventilation of patients after opioid administration.
| Meperidine | Morphine | Fentanyl | Sufentanil | Alfentanil | Remifentanil | |
|---|---|---|---|---|---|---|
| Comparative potency | 0.1 | 1 | 75–125 | 500–1000 | 10–25 | 250 |
| Peak Effect (min) | 5–7 | 20–30 | 3–5 | 3–5 | 1.5–2 | 1.5–2 |
| Duration (h) | 2–3 | 3–4 | 0.5–1 | 0.5–1 | 0.2–0.3 | 0.1–0.2 |
| Half-life (h) | 3–4 | 2–4 | 1.5–6 | 2.5–3 | 1–2 | 0.15–0.3 |
Pretreatment with midazolam has been shown to decrease episodes of muscle rigidity as well as treat the rigidity episode. The office should have a neuromuscular blocker available in case of an episode of severe rigidity. Rigidity can occur hours after the last dose of opioid has been administered. Opioids decrease the respiratory drive to increases in CO 2 . The mu receptor–stimulating opioids cause a direct depression of the respiratory center in the brainstem. Elderly patients are more sensitive to the opioid-induced respiratory depression and analgesic effect of the opioids. Opioids are associated with an increased incidence of postoperative nausea and vomiting and an antiemetic such as ondansetron (serotonin antagonist) should be considered. Fentanyl is highly lipophilic and therefore widely distributed to body tissues. The lungs show a first pass effect and take up to 75% of the IV fentanyl, which is rapidly released. Fentanyl is metabolized in the liver by N-dealkylation and hydroxylation and the primary metabolite, norfentanyl, can be found in the urine for up to 48 hours after IV fentanyl. A dose of 100 μg (0.1 mg) (2 mL) is equal to 10 mg of morphine in its analgesic effect. The onset of action is immediate and the duration of action is 30 to 60 minutes after a 2-mL dose. IV anesthesia with fentanyl injections should be initially titrated. Low-dose fentanyl 1 to 3 μg/kg IV can produce analgesia for minor painful surgical procedures. Maintenance can be achieved using nitrous oxide 50% to 60% with or without a benzodiazepine. Boluses of 25 to 50 μg every 15 to 30 minutes can be used, or an infusion pump may be used.
Fentanyl is 100 times more potent than morphine and is a pure opioid; it produces no amnesia. It has a rapid onset of less than 1 minute and a peak effect in about 2 to 3 minutes, with a duration of action of about 20 to 40 minutes. In pediatrics the IV dose is 0.5 to 1.0 μg/kg, which is titrated every 5 minutes to the desired effect, not to exceed 5 μg/kg. Similar to adults, there is chest wall rigidity and vocal cord closure that may be associated with its use or rapid administration usually in high doses. Chest wall rigidity is usually not seen with low doses of fentanyl. Patients should be observed in the recovery, because the effects on respiratory depression can be longer than the analgesic effect of fentanyl. Remifentanil is a rapid-acting opioid. It has a rapid onset and short duration of action. A high incidence of apnea and chest wall rigidity is associated with it, and its use by nonanesthesiologists is not recommended in pediatrics.
Dissociative Agents
This classification includes agents that cause interruption of cerebral association pathways between the limbic system and cortical system. It produces a catalepsylike state, in which the individual feels dissociated from the environment, and it also induces marked analgesia.
Phencyclidines (ketamine)
Ketamine produces amnesia and analgesia. It exerts its dissociative effect on the limbic/thalamic system. Ketamine is an antagonist of the NMDA receptors and an agonist of the opioid receptors. Ketamine can cause increased heart rate, cardiac output, and blood pressure. Ketamine causes bronchial smooth muscle relaxation. It improves pulmonary status in patients with reactive airway disease and bronchospasm. Ketamine also produces an associated increased salivation that can cause upper airway obstruction leading to a laryngospasm. It is not recommended for use in patients with coronary artery disease. Ketamine usually allows spontaneous respirations. It does have associated psychological effects. Ketamine has 2 isomers: S-(+) and R-(−). The S-(+) is the more potent isomer with fewer side effects. Ketamine is metabolized by the liver and its metabolite norketamine has about 30% less activity than ketamine. Ketamine produces an anesthetized state called dissociative anesthesia; patients are in a cataleptic state in which the eyes are open but they do not respond to pain. Because of its high lipid solubility, it crosses the blood-brain barrier rapidly and has a rapid onset of 30 seconds. Patients usually show pupil dilatation, nystagmus, and increased salivation. In pediatric patients the starting doses are 1 to 2 mg/kg intramuscularly and 0.25 to 1.0 mg/kg intravenously, and 4 to 6 mg/kg orally. Onset after IV administration is about 1 minute, with a duration of action of 10 to 15 minutes. After intramuscular injection, the onset is about 5 minutes and duration of action is 30 to 120 minutes.
The combination of ketamine with a benzodiazepine prolongs the effect of ketamine. There is no known antagonist of ketamine. Ketamine increases cerebral blood flow and intracranial pressure. Patients with increased intracranial pressure, such as with head trauma, should not be administered ketamine because it can further increase intracranial pressure and cause apnea. It is also contraindicated in patients with open eye injury, psychiatric disorders, as well as ischemic heart disease. Ketamine is also associated with nonpurposeful extremity movements. One of its negative aspects is the emergence phenomena seen with its use. There are illusions, fear, hyperexcitability, and what is described as an out-of-body experience. The incidence of the emergence phenomena is lower in children than in adults and is multifactorial. Midazolam and other benzodiazepines have been shown to decrease the incidence of the emergence phenomena.
As noted earlier, ketamine has not been shown to have a major effect on respiratory depression unless used in high doses. It is an excellent drug for patients with airway disease and bronchospasm because of its smooth muscle relaxation. It has been used to treat patients with resistant status asthmaticus. Although it is an excellent drug for asthmatic patients, it is associated with increased salivation that can lead to laryngospasm and silent aspiration. Ketamine is often used for pediatric sedation in the outpatient setting for dental treatment and is reported to have fewer emergent effects in children than in adults. Ketamine is usually combined with an antisialogogue such as glycopyrrolate, 0.01 mg/kg, or atropine, 0.02 mg/kg, to decrease secretions that may lead to laryngospasm.
Etomidate (Amidate)
Etomidate is often used as part of a rapid sequence induction and a modulator at the GABA receptors. GABA is a chemical messenger that inhibits the activity of brain cells. Boosting GABA levels both calms the brain and increases dopamine levels in the nucleus accumbens. It has a half-life of 75 minutes, is highly protein bound in blood plasma, and is metabolized by hepatic and plasma esterases to inactive products. Excretion is 85% in urine and 15% in the bile. It is used as an anesthetic agent because it has a rapid onset of action and a safe cardiovascular risk profile, and therefore is less likely to cause a more significant reduction in blood pressure than other induction agents. Other useful qualities of etomidate are that dosing is easy, suppression of ventilation is minimal, histamine liberation is inhibited, and it can be used safely in patients with myocardial and cerebral ischemia.
Major adverse outcomes involve corticosteroid synthesis suppression in the adrenal cortex by inhibiting 11-beta-hydroxylase, an enzyme that is important in adrenal steroid production (it leads to primary adrenal suppression). Komatsu and colleagues in 2013 conducted a retrospective study of almost 32,000 patients and found that etomidate, when used for the induction of anesthesia, was associated with a 2.5-fold increase in the risk of dying compared with those given propofol. Patients given etomidate also had significantly greater odds of having cardiovascular morbidity and significantly longer hospital stay. These results, especially given the large size of study, strongly suggest that, at the least, clinicians should use etomidate judiciously. In addition, the use of etomidate with opioids and/or benzodiazepines may potentiate etomidate-related adrenal insufficiency.
Reversal Agents and Fluids
Flumazenil
Flumazenil (Romazicon) is a competitive antagonist at the GABA A receptor and can reverse the effects of benzodiazepines. With the rapid clearance of flumazenil the possibility of resedation exists. When flumazenil is used to reverse the action of midazolam the possibility of resedation is less than when it is used to reverse other benzodiazepines because of midazolam’s rapid clearance compared with other benzodiazepines. Flumazenil reverses the respiratory depression, amnesia, and sedative effect of the benzodiazepine. Its action is rapid, with a peak effect occurring at 1 to 3 minutes. The dosage if benzodiazepine overdose is suspected is 0.1 to 0.2 mg IV to a total of 3 mg in incremental doses every 1 to 2 minutes.
In adults, a dose of 17 μg/kg has been shown to antagonize the effects of benzodiazepines, and in children a dose of 24 μg/kg has shown to have the same effect. After reversal or antagonism with flumazenil patients should be observed in the recovery room because of the possibility of resedation. Seizures have been reported when larger doses of flumazenil are used.
Naloxone
Naloxone (Narcan) is an opioid antagonist that can reverse the respiratory depression, urinary retention, rigidity, and nausea and vomiting associated with opioids. Its use is associated with an increased heart rate, increased blood pressure, and cases of pulmonary edema. Dosages range from 0.4 to 0.8 mg in adults. It has a rapid onset of action of about 1 to 2 minutes and in doses of 0.5 to 1.0 μg/kg every 2 to 3 minutes, restores spontaneous respiration.
Because of the short half-life of naloxone (30–60 minutes) renarcotization can be seen. Note that, by titration, the respiratory depression of the opioids can be reversed with little effect on the analgesia. For treating muscle rigidity associated with the opioids, both naloxone and succinylcholine may be used, with the disadvantage of naloxone reversing the analgesia opioid effect. For pediatric patients less than the age of 5 years a dose of 100 μg/kg is recommended, and for children more than 5 years old (weighing >20 kg) a dose of 2 mg of naloxone is recommended by the American Academy of Pediatrics.
Intravenous Fluids
For the purposes of office-based IV anesthesia, general practitioners use IV fluids mainly to dilute the administered anesthetic medications given to the patient. Crystalloids are used in the office setting to provide water and electrolytes as well as to expand intravascular fluid. The fluid deficit for an adult who has been fasting for 8 hours can be estimated to be 2 mL/kg for each hour before surgery. Therefore a 70-kg patient who has been nil by mouth for 8 hours has a deficit of 1120 mL. Most office dental procedures last 1 to 2 hours, and within the first hour of the procedure one-half of the deficit is replaced (560 mL), and within the second hour of the procedure one-half of the initial amount given over the first hour (280 mL) is replaced. The 4-2-1 rule for pediatric patients is often used to calculate the daily maintenance fluid requirements of 4 mL/kg/h for the first 10 kg of weight, 2 mL/kg/h for the second 10 kg of weight, and 1 mL/kg/h for each additional kilogram. Intraoperatively for pediatric patients 20 to 40 mL/kg of lactated Ringer solution may be given to replace the fluid deficit.
Crystalloids when used alone without colloids for replacement of blood volume are at a 3:1 ratio, which is 3 mL of crystalloid for every 1 mL of blood loss. Most office-based dental procedures do not require colloids for replacement therapy. Presently in the United States IV fluids are on an allocation and extremely difficult to obtain. When they are used for office-based dental anesthesia a minimum of a 1-L bag is required.
Normal saline (0.9% weight/volume) intravenous infusion blood pressure
Each 100 mL of 0.9% NaCl contains sodium chloride United States Pharmacopeia (USP) 0.9 g; water for injection. The concentration of electrolytes is sodium 154 mEq/L and chloride 154 mEq/L. It has an osmolarity of 308 mOsm/L and a pH of about 5.5. Sodium chloride for injection is sterile, isotonic, nonpyrogenic, and has no antimicrobial or bacteriostatic agents. The infusion provides fluids and electrolytes. Sodium is the major extracellular cation, whereas chloride is the major extracellular anion. Normal saline IV fluid is used for extracellular fluid replacement. Relative contraindications include patients with renal failure, congestive heart failure, and pulmonary edema. It is recommended to check the IV bag for precipitate if additional medications are added, such as antibiotic or steroids.
Dextrose 0.5% and 0.9% sodium chloride injection United States pharmacopeia
The management and sedation of diabetic patients are beyond the scope of this article; however, a fluid containing dextrose should be considered in such patients. Each 100 mL contains hydrous dextrose 5 g; sodium chloride 0.9 g; water for injection USP. It has a pH of approximately 4.4 and an osmolarity of 560 mOsm/L. It is a hypertonic solution and contains 154 mEq/L of sodium and 154 mEq/L of chloride. The dextrose provides a source of calories, and functions as free water because it is rapidly metabolized. Dextrose-containing solutions must be used with caution in patients with diabetes mellitus and clinicians must consider the patient’s potassium status because hypokalemia is a risk. Dextrose-containing solutions should also be considered for patients with a history of fainting associated with long periods of fasting.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


