This article is an update on pain management in the dental care setting for adult and pediatric patients. The 3 main categories of analgesic medications are examined: (1) opioids, (2) nonsteroidal antiinflammatory drugs (NSAIDs), and (3) nonopioid, non-NSAID medications. Pharmacology, side effects, patient selection, and treatment strategies and principles are examined. The information provided is aimed to facilitate the clinical perspective and update the oral health care clinician on providing safe and effective analgesia to adult and pediatric patients.
Key points
- •
This is an update on analgesia for acute oral pain.
- •
Analgesic medications in the dental setting are reviewed.
- •
The article includes updates for nonsteroidal anti-inflammatory drugs (NSAIDs) for dental pain.
- •
The article includes updates for opioid analgesics for dental pain.
- •
A review of analgesia for pediatric dental pain is presented.
One of the most important criteria that patients appreciate and rank a dental care provider for, far beyond knowledge and academic background, skills, and professional accolades, is effective pain management. Pain and analgesia are subjects often inherent (the former) and imperative (the latter) to oral health care. Today, despite the great scientific advances and breakthroughs in dentistry (and in its specialties) there are no real “game changers” in the world of analgesia; this fact calls for meticulous knowledge, by the clinician, of the current armamentarium and techniques to provide optimal pain control. This article navigates through this vast subject, limiting and filtering the information as to what is pertinent and useful in the dental care setting and the dental patient, adult and pediatric.
Three types of pain can be distinguished:
- •
Acute physiologic nociceptive pain that is generated by acute noxious stimuli and forms a protection from tissue damage (needle injection, incision);
- •
Pathophysiologic nociceptive pain that accompanies tissue inflammation or injury in absence of intentional stimuli (burn, postincision, local inflammation); and
- •
Neuropathic pain that is elicited from either peripheral or central disease, or injury, of neurons. It is not related to noxious stimuli and it is perceived as unnatural (diabetic neuropathy, trigeminal neuralgia).
This classification may be oversimplified because new types of pain have been described and in many cases different types coexist. A more fundamental distinction, one that is recognized even by the general public and our patients, is the one between acute and chronic pain. Chronic pain has been described as one that lasts longer than 6 months. Recently, state boards have adapted the following time frames regarding pain, to form guidelines for controlled substances:
- •
Acute pain: up to 4 weeks from onset;
- •
Postoperative pain: up to 4 weeks from date of surgery;
- •
Subacute pain: 4 to 12 weeks from onset; and
- •
Chronic pain: greater than 12 weeks from onset.
Chronic pain is more complex and the relation between nociception and pain is not linear. It may be affected by neuroendocrine dysregulation and impaired physical or mental status. Chronic pain frequently has significant effects on psychological health. Frequently, there is a direct association of chronic pain with depression and anxiety that in turn can profoundly affect pain perception.
It is beyond the purpose of this article to analyze the complex mechanisms of pain and describe the nociceptive system, yet we must recognize that a thorough understanding of those parameters will form a better and more comprehensive appreciation and selection of analgesic medications in the clinical setting.
We can consider 3 main categories of analgesics medications in the clinical dental setting:
- 1.
Opioids,
- 2.
NSAIDs, and
- 3.
Nonopioid, non-NSAID drugs.
Of course there are other categories used in the broader sense of head and neck analgesia like:
- •
Triptans (serotonin receptor agonists) that are mainly indicated for acute migraines and cluster headaches;
- •
Anticonvulsants and antidepressants that target mainly neuropathic pain that follows nerve injury; and
- •
Local anesthetics used, not for common local anesthesia, but to treat symptoms of chronic pain. Intravenously administrated local anesthetics have shown remarkable results for neuropathic pain. This indication is not relevant to the usual dental clinical setting.
Different classes of analgesic medications modulate different mechanisms and vary in their effectiveness in managing different pain states in adults ( Table 1 ). Significant pharmacodynamic and pharmacokinetic differences also exist between the adult and the pediatric populations that need to be recognized by clinicians. Here’s an outline of few differences, perhaps oversimplified:
- •
Immature blood–brain barrier in neonates may facilitate drug delivery to the brain.
- •
Lower plasma levels of albumin in neonates will result in less protein binding of drugs thus increasing action potential or even toxicity.
- •
Neonates and infants have higher body water content and thus a greater volume of distribution of water-soluble drugs, which will extend the duration of action.
- •
There are fewer pharmacodynamically inactive tissues, like muscle and adipose tissue, so there is less drug uptake by that tissue. The consequence is higher plasma concentrations.
- •
Hepatic clearance of drugs in children 2 to 6 years old may be greater compared with adults because of a larger hepatic mass relative to body weight. This may result in adjusting for relatively higher doses and shorter intervals.
- •
Renal function and excretion of drugs is reduced in neonates.
| Medication Class | Relative Effectiveness in Pain States |
|---|---|
| Nonsteroidal antiinflammatory drugs | Tissue injury >> acute stimuli = nerve injury = 0 |
| Opioids (μ agonists) | Tissue injury = acute stimuli > nerve injury > 0 |
| Anticonvulsants | Nerve injury > tissue injury = acute stimuli = 0 |
| Antidepressants | Nerve injury > tissue injury >> acute stimuli = 0 |
Another important, yet understated, factor in pediatric patients is pain assessment and evaluation. Older children (school age) can usually perceive and convey an adequate pain self-assessment especially with the help of visual analog scales. The challenge lies with younger children and children with cognitive delay. For those children, third-party assessment tools are necessary. To add to the challenge, it is documented that third-party assessment is inferior to self-assessment of pain intensity. There are many pain scales that can be used by parents or clinicians. One example is the FLACC (face, legs, activity, cry, consolability) scale shown in Table 2 . It is designed for children between 2 months and 7 years of age and can be used even in children who are unable to speak. The range is 0 to 10 with 0 representing no pain.
| Parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, uninterested | Frequent to constant quivering chin, clenched jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking, or legs drawn up |
| Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers: occasional complaint | Crying steadily, screams or sobs, frequent complaints |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or being talked to, distractible | Difficult to console or comfort |
The major categories of analgesics are presented next. In this article, only drugs currently approved by the US Food and Drug Administration, for use in United States, are discussed.
Opioid analgesics
When it comes to severe pain the most effective and broadly used drugs are opioids. Three classes of true opioid receptors (μ, δ, and κ) have been described with multiple receptor subtypes.
-
μ (mu) – The μ opioid receptors produce the most profound analgesia, and can cause euphoria, respiratory depression, physical dependence, and bradycardia. They are responsible for most of the analgesic effect of the opioid.
-
κ (kappa) – The κ opioid receptors contribute to analgesia at the spinal level. These receptors trigger a lesser analgesic response, and may cause miosis, sedation, and dysphoria.
-
δ (delta) – The δ opioid receptors modulate μ receptor activity and are more important in the periphery.
Additionally, the following receptors have been associated with opioids but are not consider as true opioid receptors:
-
σ (sigma) – The σ opioid receptors can be stimulated by opioids and may account for the excitatory actions of opioids; however, these excitatory effects probably are produced by an interaction with the phencyclidine binding site on ionotropic glutamate receptors.
-
Orphan opioid receptor-like receptors are structurally similar to the μ opioid receptor, but are insensitive to opioid ligands.
The pathways of opioid receptor signaling are multiple, like G-protein coupling, cyclic adenosine monophosphate inhibition, and Ca ++ channel inhibition; a detailed analysis of these agents is beyond the purpose of this article. The opioid effect is a selective one on nociception. Touch, pressure, and other sensory modalities are generally unaffected.
All known use of opioid medications goes back in history thousands of years and it starts with the plant Papaver somniferum. The dry exudate of the plant’s seed pod is opium , which is a complex mix that includes alkaloids like morphine, codeine, noscapine, papaverine, and thebaine. These alkaloids have been used traditionally in medicine to treat pain, cough, and visceral spasms. Morphine is recognized as the standard opioid.
Opioids can be used in both acute and chronic severe pain. Acute situations include intraoperative, postoperative, and posttraumatic pain. The application can be preemptive, before the noxious stimulus, like the administration of intravenous (IV) opioids with anesthetic agents before surgery, or therapeutic after the noxious stimulus. Chronic conditions where opioids can be considered should be divided into:
- A.
Malignant (cancer pain) that is responsive to opioids.
- B.
Nonmalignant (like neuropathic or inflammatory pain). This type will be better addressed in a multidisciplinary way with both pharmacologic and nonpharmacologic treatments.
In general, long-term use of opioids for chronic conditions falls outside of the subject matter of most dental care facilities.
Opioid Pharmacodynamics
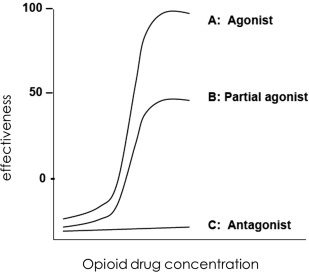
Opioids can be agonists, partial agonists, or antagonists ( Fig. 1 ). Drug A is a drug that binds and produces a concentration-dependent activation of the receptor is an agonist. Drug B is a partial agonist in relation to drug A. Drug C binds to the receptor yet elicits no effect is an antagonist. By far the most efficacious and widely used clinically drugs are the μ-receptor agonists.
Opioid Pharmacokinetics
Absorption after oral administration is rapid and first-pass metabolism at the liver is significant. Because of the rapid absorption, sustained-release opioid formulations have been developed to achieve adequate duration of analgesia. The lipophilicity of the formulation also increases the bioavailability. Lipophilic drugs can be absorbed from mucosal surfaces into the bloodstream; hence, opioids are now available commercially in buccal, intranasal, and transdermal formulations. Distribution is speedy throughout the body. In the case of peripheral tissue injury, like surgical trauma, up to 80% of an analgesic effect after systemic opioid administration may be mediated by peripheral opioid receptors.
All clinically available opioids undergo extensive hepatic metabolism to metabolites that are excreted by the kidneys. The only exception is remifentanil (not available for oral administration), which is rapidly hydrolyzed by esterases in peripheral tissues and plasma.
Side Effects
- •
Cardiovascular: bradycardia in high doses.
- •
Respiratory: dose-dependent respiratory depression. This is a direct effect on the respiratory center in the medulla. High doses can produce apnea.
- •
Gastrointestinal: opioids cause inhibition of normal intestinal secretions and peristalsis that can lead to increased water absorption and constipation.
- •
Central: nausea and vomiting stimulation by a direct effect in the brainstem.
- •
Central: cough suppression by direct effects on medullary cough centers.
- •
Central: pupil constriction (miosis) by a direct effect on the autonomic nucleus (Edinger–Westphal) of the oculomotor nerve.
- •
Central: euphoria and sedation in higher doses.
- •
Histamine release from tissue mast cells is usually associated with IV administration. Local itching, redness, or hives are observed near the site of injection and do not represent an allergic reaction because true allergy to opioids is extremely rare.
- •
Tolerance after repeated or prolonged exposure. This affects more the analgesic effect.
- •
Physical dependence: abrupt discontinuation of opioids causes a stereotypical withdrawal syndrome. Another dimension to this phenomenon is the psychological dependence. Addiction liability is apparently very low in chronic pain patients.
Management suggestions for some of those side effects are illustrated in Table 3 .
| Side Effects of Opioids | Examples of Pharmacologic Management |
|---|---|
| Constipation | Osmotic laxative: magnesium citrate |
| Softening agent: sodium docusate | |
| Cathartic: bisacodyl, senna | |
| Nausea and vomiting | Neuroleptic: prochlorperazine |
| Antihistamines: promethazine, diphenhydramine, meclizine | |
| Prokinetics: metoclopramide | |
| Pruritus, hives | 5-HT3 receptor antagonist: ondansetron |
| Antihistamines: diphenhydramine |
Opioid agents can be used for pain experienced as moderate to severe. In this article, agents that are administered orally and/or may be considered for analgesia in the dental care setting are highlighted. A few others are listed as honorable mentions. A comparison of various pharmacokinetic parameters can be found in Table 4 . Adult dosing is found in Table 5 .
| Opioid | Route | Onset of Action (min) | Time to Peak Effect (min) | Duration of Action (h) |
|---|---|---|---|---|
| Morphine | Oral | 60 | 60–120 | 4–5 |
| Fentanyl | Intravenous | 1–2 | 3–5 | 0.5–1 |
| Hydromorphone | Oral | 30 | 90–120 | 4 |
| Levorphanol | Oral | 10–60 | 90–120 | 4–5 |
| Meperidine | Oral | 15 | 60–90 | 4–6 |
| Codeine | Oral | 30–40 | 60–120 | 4 |
| Hydrocodone | Oral | 10–30 | 30–60 | 4–6 |
| Oxycodone | Oral | 10–30 | 60 | 3–4 |
| Pentazocine | Oral | 15–30 | 30–60 | 2–3 |
| Tramadol | Oral | 30–40 | 120 | 6 |
| Opioid | Oral Dose for Severe Pain in Adults |
|---|---|
| Morphine (MS Contin, Avinza, Kadian) | 15–30 mg q3-4 h (short acting) |
| Dosages may vary for long-acting formulations | |
| Codeine (generic) | 15–60 mg q4 h |
| Codeine (with acetaminophen) (Tylenol #3) | 15–60 mg q4 h |
| Hydrocodone (with acetaminophen) (Vicodin) | 5–7.5 mg q4-6 h |
| Oxycodone (Oxycontin) | 5–15 mg q4-6 h |
| Oxycodone (with acetaminophen) (Percocet) | 2.5–10 mg q4-6 h |
| Hydromorphone (Dilaudid) | 2–8 mg q3-6 h |
| Levorphanol (Levo-Dromoran) | 2–3 mg q6-8 h |
| Meperidine (Demerol) | 50–150 mg q3-4 h |
| Pentazocine (Talwin NX) | 50 mg q3-4 h (contains also naloxone) |
| Tramadol (Ultram) | 50–100 mg q4-6 h. MAX 400 mg/d |
Regarding the pediatric patient population, in the dental care setting there are few and distinct instances where the need for opioids would emerge to manage moderate to severe nociceptive pain. In such cases careful titration is required to obtain the desired level of analgesia while monitoring side effects. Table 6 provides dosing for children.
| Opioid Medication | Starting Dose for Children (Oral) |
|---|---|
|
1–2 y: 200–400 μg/kg q4 h 2–12 y: 200–500 μg/kg q4 h (max 5 mg) |
|
200–800 μg/kg q12 h |
|
30–80 μg/kg q3–4 h (max 2 mg/dose) |
|
125–200 μg/kg q4 h (max 5 mg/dose) |
|
5 mg q12 h |
| Hydrocodone | 100–200 μg/kg q4 h |
| Codeine | 0.5–1 m/kg q4 h |
| Tramadol | 1–2 mg/kg q6 h (max per dose: 100 mg max per day 400 mg) |
Morphine
Morphine is the quintessential opioid, the drug that best represents the aforementioned pharmacology. Generic and multiple trade formulations are available and it can be also given by mouth usually as an elixir. Bioavailability is approximately 30%. Larger oral doses are needed to produce equivalent analgesia with parenteral administration with the ratio being 3:1. The pharmacokinetics of morphine in children are comparable with those in adults. Neonates, however, differ, because morphine doses need to be reduced owing to hepatic immaturity. Examples of brand names are MS Contin, Avinza, Kadian, and Roxanol.
Methadone
Methadone is best known for its use in treating narcotic addiction. It is not recommended in the dental care setting and without significant expertise.
Meperidine
Meperidine or pethidine is well-absorbed from gastrointestinal track with a bioavailability reaching 50% to 60%. It crosses the placenta and also enters breast milk. It has fallen out of favor, in relation with past use, mostly because of its neurotoxic metabolite, norpethidine, Meperidine has demonstrated local anesthesia ability, particularly in spinal anesthesia, so local administration has being explored intraorally for anesthesia and analgesia without encouraging outcomes. Examples of brand names are Demerol and Meperitab.
Fentanyl
Fentanyl is available in transdermal extended release formulation and sublingual spray, both prescribed by pain specialists to chronic pain patients. The IV form, however, may be encountered in the dental care outpatient setting because qualified practitioners may administer it during IV sedation.
Remifentanil
Remifentanil is a potent, ultra–short-acting opioid that is also used in the outpatient IV sedation setting. It works best when combined with another agent (like propofol), especially using computer controlled infusion pumps.
Naloxone
Naloxone (Narcan) is an μ-opioid receptor competitive antagonist, and is usually administered parenterally to counter the effects of opioid overdose. It has no analgesic effects.
Codeine
Codeine is a weak μ opioid agonist used as an analgesic and as an antitussive. Codeine is considered a prodrug because it is metabolized in vivo to the primary active compounds morphine and codeine-6-glucuronide. This metabolism of codeine takes place in the liver through the actions of CYP2D6 enzyme. Most patients have normal CYP2D6 activity and their codeine response is normal. A substantial minority of patients, however, expresses genetic polymorphism that is racially and ethnically influenced and has CYP2D6 activity that is higher or lower than normal.
- a.
Higher activity means increased conversion to morphine and excessive sedation, constipation and side effects. Ultrarapid metabolizers are found in about 1% of people from Finland and Denmark, about 4% in Caucasian North Americans, about 10% of people from Greece and Portugal, about 20% in Saudi Arabia, and almost 30% of people from Ethiopia.
- b.
Lower activity means that codeine is unlikely to be an effective painkiller. Total CYP2D6 deficiency occurs in about 6% to 10% of Caucasians, 3% to 6% of Mexican Americans, 2% to 5% of African Americans, and about 1% of Asian Americans.
Also, people with normal CYP2D6 activity may be taking drugs that substantially inhibit CYP2D6, rendering codeine ineffective ( Table 7 ). Because of case reports involving morbidity and mortality owing to the use of codeine in children with higher enzymatic activity, the European Medicines Agency restricted the use of codeine to children over 12 years of age. Additionally, the US Food and Drug Administration issued a boxed warning in 2012 regarding avoiding codeine for postoperative pain in children undergoing tonsillectomy. The warning says that, “for management of other types of pain in children, codeine should only be used if the benefits are anticipated to outweigh the risks.” Dental pain may very well fit into this description.
| Generic Name | Brand Name |
|---|---|
| Amiodarone | Cordarone |
| Bupropion | Wellbutrin |
| Chloroquine | Aralen |
| Cinacalcet | Sensipar |
| Diphenhydramine | Benadryl |
| Fluoxetine | Prozac |
| Haloperidol | Haldol |
| Imatinib | Gleevec |
| Paroxetine | Paxil |
| Propafenone | Rythmol |
| Quinidine | Quinidex |
| Terbinafine | Lamisil |
| Thioridazine | Mellaril |
The most common formulation is in combination with acetaminophen. Examples of brand names are Tylenol #3 and Tylenol #4.
Oxycodone
Oxycodone is a weak opioid agonist used either alone or in combination with NSAIDs or acetaminophen for analgesia. Because oxycodone does not rely on active metabolites there are no considerations regarding enzymatic activity addressed previously for codeine. Examples of brand names include:
- •
Plain: Oxycontin, Roxicodone;
- •
With acetaminophen : Percocet, Roxicet, Narvox;
- •
With aspirin: Percodan, Endodan; and
- •
With ibuprofen: Combunox.
Hydrocodone
Hydrocodone is a weak opioid agonist used in combination with NSAIDs or acetaminophen for analgesia. Hydrocodone shares the same considerations regarding enzymatic activity addressed previously for codeine. The plain form is formulated as extended release and is used for chronic pain. It can be found also in cough suppressing drug combinations. Examples of brand names include:
- •
Plain: Hysingla extended release, Zohydro extended release;
- •
With acetaminophen : Vicodin, Lorcet, Lortab, Hycet; and
- •
With ibuprofen: Vicoprophen, Ibudone, Reprexain.
Pentazocine
Pentazocine is a κ opioid receptor agonist and a μ opioid receptor antagonist. Its analgesic effects are subject to a ceiling effect. Pentazocine is more likely to cause hallucinations and other psychotomimetic effects. It is provided in combination with naloxone. Naloxone is not orally bioavailable and it produces no effect when administered orally, but it blocks the opioid effects of pentazocine if injected intravenously for recreational purposes. Examples of brand names include Talwin NX.
Hydromorphone
Hydromorphone is a μ-opioid agonist and is better absorbed orally than morphine. It is also faster acting and more potent that morphine. Examples of brand names include Dilaudid and Exalgo.
Propoxyphene (Darvon)
Propoxyphene (Darvon) has been discontinued since 2010.
Tapentadol
Tapentadol is a recently approved medication with a dual mechanism: μ-opioid receptor agonist and norepinephrine reuptake inhibitor. It has equal analgesic effect, with a lower incidence of side effects, when compared with morphine and oxycodone. The potential for dependency is not fully clarified yet emerging data are favorable. Brand names include Nucynta.
Tramadol
Tramadol is a synthetic substance that shows both opioid and nonopioid properties. It is an atypical opioid that structurally is related to codeine, but it is 10 times less potent. Tramadol undergoes hepatic metabolism that is dependent on CYP2D6. It is a weak agonist for μ-opioid and κ-opioid receptors and a norepinephrine and serotonin reuptake inhibitor. Owing to this monoaminergic action, may also exhibit antidepressant and anxiolytic-like effects. The advantage of tramadol is that it displays a lower incidence of side effects and abuse potential. It can be considered a preferable choice for pain relief compared with NSAIDs because their long-term use may lead to impairment of renal function and cause gastrointestinal complications, and with respect to other opioid medications for its low addiction rate and favorable safety profile. The most troubling adverse effect of tramadol is nausea and vomiting, especially with oral administration. Also, caution should be exercised in patients with seizure disorder. Metabolizes in the liver. As of August of 2014 the Drug Enforcement Administration has placed Tramadol into schedule IV of the Controlled Substances Act. Examples of brand name include Ultram, Ryzolt, ConZip, and Ultracet (combined with acetaminophen).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


